Pharmaceutical and Life Sciences Real World Evidence Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Pharmaceutical and Life Sciences Real World Evidence Market is Segmented By Type of Applications (Ea...
Pharmaceutical and Life Sciences Real World Evidence Market Size - Analysis
The Pharmaceutical and Life Sciences Real World Evidence Market is estimated to be valued at USD 2.0 billion in 2024 and is expected to reach USD 5.3 billion by 2031, growing at a compound annual growth rate (CAGR) of 15% from 2024 to 2031.
The market is witnessing positive trends owing to rise in usage of real-world data to assess relative effectiveness and safety of treatment options. Pharmaceutical companies are increasingly leveraging real world evidence to expedite clinical development, better target patient populations, and improve health outcomes.
Market Size in USD Bn
CAGR15%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 15% |
| Market Concentration | Medium |
| Major Players | Clinerion, Clinigen Group, Cognizant Analytics, Evidera, HealthCore and Among Others |
please let us know !
Pharmaceutical and Life Sciences Real World Evidence Market Trends
Market Driver - Increasing Adoption of Real-World Evidence in Regulatory Decisions
The use of real-world evidence in regulatory decision-making is being increasingly adopted. Regulators around the world are showing more openness to using real world data generated outside of traditional clinical trials. They recognize that real world evidence can help address some key limitations of randomized controlled trials like limited sample sizes, restricted patient populations and controlled environments. It provides a more pragmatic view of how medical products and interventions perform during routine clinical use.
In the United States, the FDA has published several guidance in the past few years clarifying their view on using real world evidence for regulatory purposes. This includes guidance on using real world data to support labeling changes and drug approval. The FDA sees real world evidence as complementing other sources of evidence like randomized trials. It believes real world data when generated using robust methodologies can help support various decisions throughout the product lifecycle including new target identification, safety surveillance and clinical use. In the EU, regulators have also acknowledged real world evidence can potentially support marketing authorization under exceptional circumstances when clinical trial data is difficult to obtain.
The growing adoption by regulators stems from recognition that real world evidence studies can address certain gaps and limitations of traditional clinical research methods. It provides insights into treatment patterns, adverse events, effectiveness and other outcomes during routine medical practice.
Market Driver - Rising Healthcare Expenditure on Real World Data Analysis
Healthcare costs continue to rise significantly across both developed and developing countries. This exerts tremendous financial pressure on governments and private insurers to curb spending and optimize available resources. At the same time, there is a growing push for more evidence-based healthcare practices and performance benchmarking of various treatment options. This has led to greater focus on health technology assessment and analyzing real world performance or medical interventions, drugs and devices.
Healthcare payers and insurers are showing increased interest in real world evidence studies to evaluate the value and economic outcomes of various treatments. Real world data generated during routine clinical practice provides insights into effectiveness, safety, quality of life outcomes and economic impacts like costs of associated hospitalizations, Lost work productivity etc. during naturalistic use. Such data helps payers and insurers make more informed decisions about formulary inclusion, reimbursement rates and covered benefits for different treatment options. It allows them to negotiate effectively with life sciences companies and ensure value for money for funded healthcare services.
Given rising cost of healthcare, governments and private insurers also want improved cost-effectiveness and performance benchmarking across healthcare providers. Real world evidence analysis allows monitoring of cost and quality metrics during routine operations. It helps identify unwarranted variations, evaluate different care delivery models, and helps scale up more efficient practices. This in turn supports performance-driven reimbursement and more outcomes-based healthcare funding. Overall, to curb rising costs and optimize resource allocation, real world data analysis is expected to see increasing expenditure support from governments, private insurers and healthcare payers.
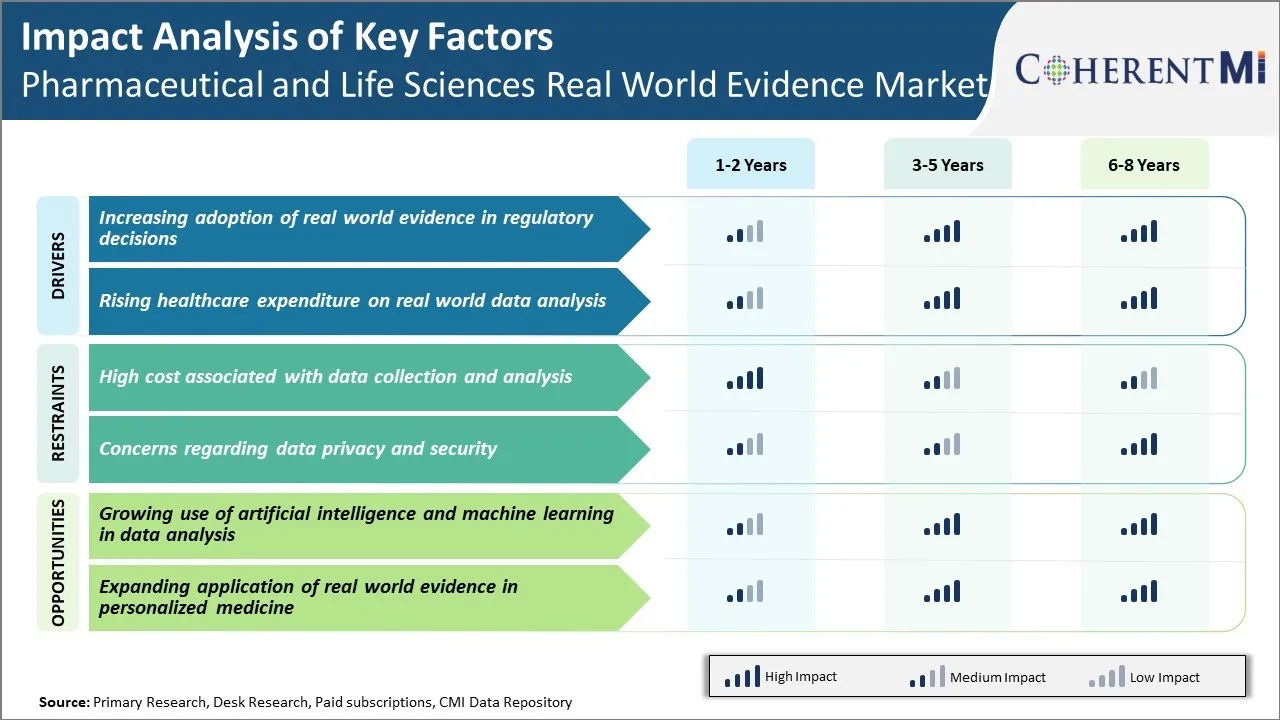
Market Challenge - High cost associated with data collection and analysis
One of the major challenges faced by the pharmaceutical and life sciences sector regarding real world evidence is the high cost associated with data collection and analysis. Gathering real world data from electronic health records, claims databases, registries and other sources is an expensive process as it requires building the necessary infrastructure and partnerships to access these datasets. It also involves overcoming various regulatory and privacy hurdles regarding the usage of patient health information. Additionally, analyzing the vast amounts of real-world data gathered from multiple sources in an effective manner requires heavy investments in data management and analytics tools, as well as hiring skilled data scientists and researchers to derive meaningful insights. Linking disparate data sources across different organizations and geographies further adds to the complexity and expense of real-world data collection and analysis for the life sciences industry. The costs incurred do not always guarantee successful outcomes from real world evidence studies, making return on investment difficult to ascertain for pharmaceutical companies. Overall, the expenditure required to generate real world evidence from real world patient data poses significant budget constraints on life sciences organizations, especially smaller to mid-sized firms.
Market Opportunity: Growing Use of Artificial Intelligence and Machine Learning in Data Analysis
One major opportunity for the pharmaceutical and life sciences real world evidence market lies in the growing application of artificial intelligence and machine learning techniques for data analysis. As real-world datasets continue expanding in size and complexity, traditional statistical methods are reaching their limitations in effectively studying these massive real-world databases. Advanced technologies like deep learning, natural language processing and predictive analytics offer novel ways to extract valuable insights from the sea of unstructured, multi-dimensional patient information. AI capabilities like automated pattern recognition, segmentation and outcome prediction can help analyze real world data at faster speeds and larger scales than traditional human-driven approaches. This will enable deriving clinically relevant findings in more efficient, cost-effective ways to support drug development and outcomes research. As life sciences firms increasingly invest in AI to optimize their R&D pipelines, they are also exploring ways to leverage these technologies for real world evidence generation. The integration of AI stands to transform how real-world patient data is studied to accelerate medical progress.
Key winning strategies adopted by key players of Pharmaceutical and Life Sciences Real World Evidence Market
Major players like IQVIA and IBM Watson Health have built huge databases by aggregating real-world data from electronic health records, medical claims, patient registries and other sources. For example, IQVIA's Quintiles Real-World Evidence & Insights (RWE&I) database includes over 400 million patient records, 200 billion clinical measurements and 1 billion pharmaceutical dispensations. Such extensive databases give these players a competitive edge by enabling deep analytics capabilities for drug safety and efficacy studies.
Players are forming strategic partnerships and alliances to expand data access and scope of services. For example, in 2018, Optum partnered with Epic to gain insights from Epic's electronic health record data on over 200 million patients. This enhanced Optum's database by over 40% virtually overnight. Similarly, PPD partnered with Flatiron Health in 2017 to integrate data from oncology clinics into its evidence generation platform. Such partnerships have helped players strengthen their value proposition.
Leaders like IQVIA have diversified from traditional CRO services into a full suite of real-world evidence solutions like protocol development, study feasibility analysis, data management and reporting. This one-stop-shop approach has improved customer stickiness in a consolidating market. For example, after initial pilot projects with IQVIA, Merck signed $1 billion+ long-term strategic agreements in 2019 to jointly develop evidence solutions.
Segmental Analysis of Pharmaceutical and Life Sciences Real World Evidence Market
Insights, By Type of Applications: Early-Stage Research Sub-segment Dominates Applications Segment
Early-stage research sub-segment contributes the highest share of 25.7% in the pharmaceutical and life sciences real world evidence market due to the growing need for real-world insights during drug development. Real-world evidence plays a vital role in guiding researchers as they work to identify new drug targets and develop novel therapies. Traditional clinical trials have limitations in capturing the heterogeneity seen in patient populations in real-world settings. Real-world data provides a more comprehensive view of patient populations, disease progression, treatment patterns, and outcomes across diverse demographic and geographic groups. This level of diversity and scale is invaluable during early research as scientists work to develop solutions tailored to address the needs of subgroups that may be underrepresented in trials.
Additionally, real-world evidence can help validate candidate drug targets and biomarkers earlier in the process, potentially saving resources that would otherwise be spent on candidates unlikely to succeed in later stages of development. By leveraging real-world data's ability to provide real-time, longitudinal insights into patient journeys, the early research segment benefits greatly from this innovative data source.

Insights, By Type of Real-World Data Sources: Medical Claims Sub-segment Dominate Real World Data Sources Segment
The medical claims sub-segment contributes the highest share of 28.6% in the pharmaceutical and life sciences real world data sources market due to its established architecture for collecting longitudinal patient data. Medical claims capture detailed information on patient diagnoses, procedures, prescriptions, and costs of care over extended periods. This comprehensive view of a patient's healthcare journey and treatment outcomes over time makes claims a rich source of insights. The scale and structure of claims data also lend it significant advantages over other sources - insurers and public programs collect claims for large patient populations nationally. This allows analysis of rare conditions and subpopulations not feasible with smaller data sets.
Additionally, the financial incentives inherent in healthcare reimbursement have motivated ongoing investments to digitize and centralize claims over decades. As a result, health plans and providers have amassed big data infrastructure ideal for advanced analytics. Other real world data sources are growing, but the established foundation and scale of claims data will continue driving its primary role in powering real-world evidence applications.
Additional Insights of Pharmaceutical and Life Sciences Real World Evidence Market
- The real-world evidence solutions market is poised for substantial growth, driven by the increasing adoption of real-world data in regulatory decisions and healthcare decision-making processes. Pharmaceutical companies are investing significantly in generating real world evidence to support clinical development, with large firms spending around $20 million annually. The integration of advanced tools and analytical algorithms is enhancing the utility of real-world data, particularly in understanding the clinical value of new treatments.
- Real world evidence has the potential to save up to USD 1 billion per year by complementing results from controlled clinical trials.
- Pharmaceutical companies spend nearly USD 20 million annually on generating real world evidence for clinical development programs.
Competitive overview of Pharmaceutical and Life Sciences Real World Evidence Market
The major players operating in the Pharmaceutical and Life Sciences Real World Evidence Market include Clinerion, Clinigen Group, Cognizant Analytics, Evidera, HealthCore, IBM, ICON, IQVIA, Medpace, NorthWest EHealth, Optum Insight, Oracle, PAREXEL, Perkin Elmer, SAS, Syneos Health, and TriNetX.
Pharmaceutical and Life Sciences Real World Evidence Market Leaders
- Clinerion
- Clinigen Group
- Cognizant Analytics
- Evidera
- HealthCore
Pharmaceutical and Life Sciences Real World Evidence Market - Competitive Rivalry

Pharmaceutical and Life Sciences Real World Evidence Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Pharmaceutical and Life Sciences Real World Evidence Market
- In September 2023, HealthVerity launched HealthVerity Audience Manager, enhancing insights into patient behavior and social determinants of health.
- In July 2023, nference collaborated with Vanderbilt University Medical Center to advance real world evidence generation.
- In July 2023, Thermo Fisher Scientific acquired CorEvitas for USD 912.5 million to strengthen its regulatory grade real world evidence.
Pharmaceutical and Life Sciences Real World Evidence Market Segmentation
- By Type of Applications
- Early-Stage Research
- Clinical Development
- Regulatory Approval
- Pricing / Reimbursement
- Post Approval Studies
- By Type of Real-World Data Sources
- Medical Claims
- Clinical Trials
- Clinical Setting
- Patient Powered
- Others

Would you like to explore the option of buyingindividual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Frequently Asked Questions :
What are the key factors hampering the growth of the Pharmaceutical and Life Sciences Real World Evidence Market?
The high cost associated with data collection and analysis and concerns regarding data privacy and security are the major factors hampering the growth of the Pharmaceutical and Life Sciences Real World Evidence Market.
What are the major factors driving the Pharmaceutical and Life Sciences Real World Evidence Market growth?
The increasing adoption of real-world evidence in regulatory decisions and rising healthcare expenditure on real world data analysis are the major factors driving the Pharmaceutical and Life Sciences Real World Evidence Market.
Which is the leading Type Of Applications in the Pharmaceutical and Life Sciences Real World Evidence Market?
The leading Type of Applications segment is Clinical Development.
Which are the major players operating in the Pharmaceutical and Life Sciences Real World Evidence Market?
Clinerion, Clinigen Group, Cognizant Analytics, Evidera, HealthCore, IBM, ICON, IQVIA, Medpace, NorthWest EHealth, Optum Insight, Oracle, PAREXEL, Perkin Elmer, SAS, Syneos Health, and TriNetX are the major players.
What will be the CAGR of the Pharmaceutical and Life Sciences Real World Evidence Market?
The CAGR of the Pharmaceutical and Life Sciences Real World Evidence Market is projected to be 15.0% from 2024-2031.