Viral Clearance Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Viral Clearance Market is Segmented By Type of Scale of Operation (Discovery Phase, Preclinical Phase, Clinical Phase), By Type of Method of Viral Cle....
Viral Clearance Market Size
Market Size in USD Bn
CAGR10%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 10% |
| Market Concentration | Medium |
| Major Players | Charles River Laboratories, Creative Biolabs, Eurofins Scientific, Microbac Laboratories, MilliporeSigma and Among Others. |
please let us know !
Viral Clearance Market Analysis
The Viral Clearance Market is estimated to be valued at USD 0.9 Bn in 2024 and is expected to reach USD 1.7 Bn by 2031, growing at a compound annual growth rate (CAGR) of 10% from 2024 to 2031. The market has seen steady growth over the past few years driven by increasing demand for developing safe and effective biologics and therapeutics.
The market is expected to witness positive growth over the forecast period supported by rising prevalence of viral infectious diseases worldwide and increasing R&D investments in biopharmaceutical industry for drug development. Furthermore, stringent regulatory guidelines regarding safety of biotherapeutics and growing adoption of viral clearance testing during drug development and manufacturing is expected to supplement the market growth during next few years.
Viral Clearance Market Trends
Market Driver - Increasing Demand for Viral Clearance Testing Due to The Rise in Biologics and Biopharmaceuticals
The demand for viral clearance testing has witnessed significant growth over the past decade primarily due to the increasing production and commercialization of complex biologics and biopharmaceuticals. Biologics such as monoclonal antibodies, recombinant proteins, gene and cell therapies have revolutionized the treatment of several chronic and life-threatening diseases. However, biologics are derived from or manufactured using living cells and organisms, which makes them highly susceptible to viral contamination during upstream and downstream processing. Even trace amounts of virus can potentially contaminate large batches, thereby affecting patient safety. Considering the vulnerable patient populations targeted by these life-saving therapies, pharmaceutical companies and regulators have tightened quality standards requiring exhaustive testing at multiple stages to detect and eliminate viral impurities.
Furthermore, the manufacturing of biologics involves complex, multi-step production processes compared to conventional small molecule drugs. Live cell-based platforms and expression systems are commonly utilized during upstream development and bulk manufacturing. This increases the risk of introduction, persistence or reactivation of viruses. Additionally, biologics production frequently relies on primary human or animal cells and tissues, which can harbor endogenous retroviruses and other pathogens. All these factors mandate extensive viral characterization, validation and clearance studies throughout the development lifecycle to assure product purity and safety. The large batch sizes and strictly regulated release specifications for biologics imply that even a single production run failure can have significant financial implications for manufacturers. Therefore, viral safety testing forms a crucial part of the rigorous quality control processes established by organizations.
Market Driver - Advancements in Viral Clearance Technologies Such as Next-Generation Sequencing
The viral clearance market has witnessed remarkable transformation driven by rapid progress in next-generation sequencing (NGS) based technologies. Traditionally, PCR and electron microscopy formed the mainstay analytical solutions for viral detection and identification during product purification and characterization. However, these approaches have limited sensitivity to uncover trace contaminants or discover novel viruses. They are also incapable of full genomic fingerprinting required for conclusive viral characterization. On the other hand, new generation sequencing overcomes several bottlenecks of conventional methods through its ability to generate high volumes of sequence data from complex viral metagenomes. Advanced sample preparation coupled with massively parallel sequencing readout enables comprehensive viral screening with unparalleled depth and breadth.
NGS assays have revolutionized viral clearance capabilities through unmatched multiplexing and low detection limits in the range of few viral copies. They can simultaneously test for a broad panel of known viruses as well as discover new or variant pathogens during process optimization, characterization and product release. Using sequence data and viral genome maps as fingerprints, even highly divergent or genetically modified viruses can be accurately identified. It also facilitates high resolution genomic analysis, strain tracking and mutation surveillance and supports detailed root cause investigations in case of any detections. With continually declining costs, scalable NGS workflows are progressively replacing traditional techniques in major biomanufacturing hubs. Their implementation assures future-proof viral characterization aligned with rapidly evolving industry and regulatory expectations.
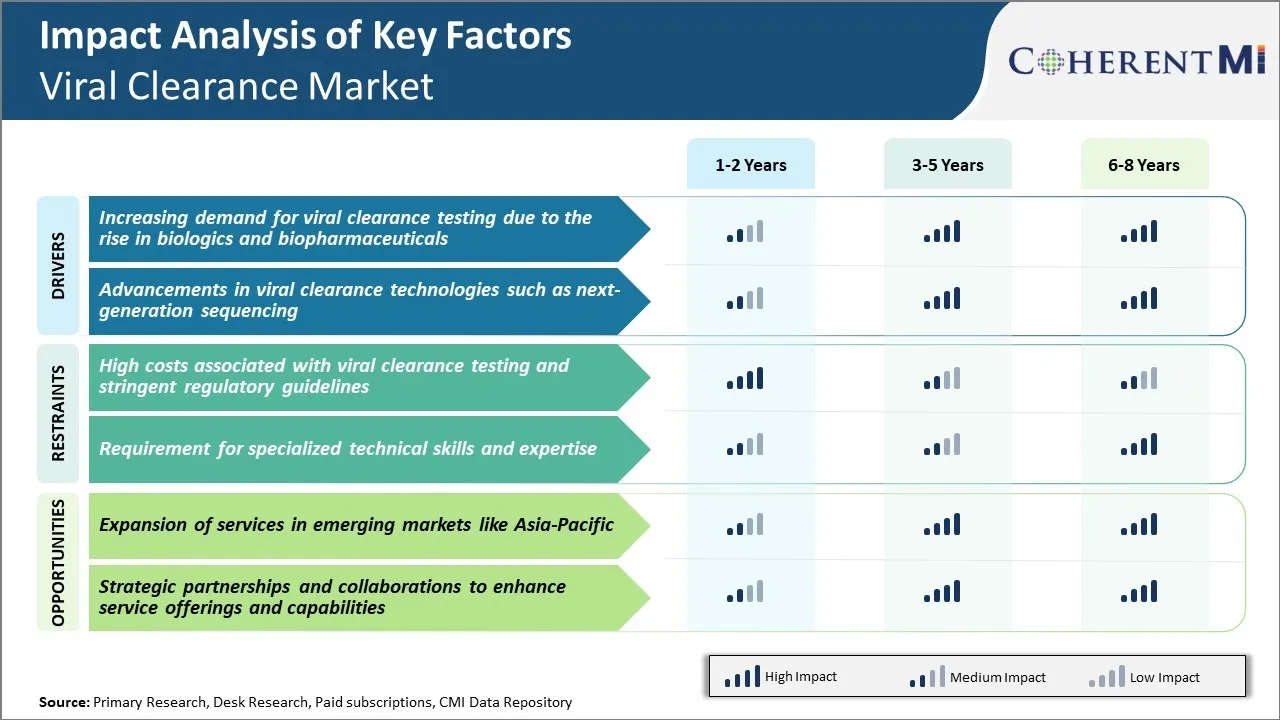
Market Challenge - High Costs Associated With Viral Clearance Testing and Stringent Regulatory Guidelines
The viral clearance market is facing many challenges due to the high costs associated with viral clearance testing as well as stringent regulatory guidelines. Viral clearance testing requires specialized equipment and biosafety facilities to handle live viruses, which contributes to significantly high capital and operational costs for companies providing these services. Additionally, the testing process involves multiple analytical techniques like PCR, ELISA, etc. to accurately detect and remove viruses, making the overall process very expensive. This high cost is ultimately passed on to biopharmaceutical clients in the form of high service charges. Moreover, stringent regulations from regulatory bodies like FDA and EMA have made the approval process longer and more complex. Companies need to present extensive data from well-designed viral validation studies as per ICH guidelines to get their products approved. This stringent regulatory oversight has increased compliance burden for market players. With rising R&D investments of biopharmaceutical firms, the testing costs are negatively impacting their profit margins.
Market Opportunity: Expansion of Services in Emerging Markets Like Asia-Pacific
There is a major opportunity for viral clearance service providers to expand their services in emerging Asian markets like India, China, South Korea and others. The Asia-Pacific region represents one of the fastest growing pharmaceutical markets globally with increasing biologics production by local firms. There is significant outsourcing of bioanalysis and preclinical safety testing to Asia by large multinational bio-companies due to lower costs. This presents a large untapped potential for viral clearance testing companies. By setting up testing facilities and partnering with local testing companies in these Asian countries, global viral clearance players can leverage lower operating costs and tap into the growing client demand. Several Asian governments are also implementing favorable policies to promote local biosimilar production and R&D. This adds to the growth prospects of the viral clearance market in Asia-Pacific region.
Key winning strategies adopted by key players of Viral Clearance Market
Innovation has been one of the most successful strategies adopted by leading players. For instance, Merck KGaA heavily invests in R&D, with over 10% of its annual revenue allocated towards it. In 2016, it introduced a cutting-edge platform called ParVivo that uses baculovirus for viral clearance studies. This helped Merck strengthen its portfolio of viral safety testing solutions.
Players have partnered with academic institutions and biotechs to gain access to new technologies and stay ahead of the competition. For example, in 2018, Charles River Laboratories signed agreements with multiple partners to incorporate new methods like high-throughput sequencing within its drug discovery services. This expanded Charles River's service capabilities.
To tap into high growth opportunities, players have expanded their geographic footprint in emerging nations like China, India and South Korea through acquisitions and organic means. In 2017, Texcell acquired JV MediCell increasing its presence across Asia Pacific. This expanded Texcell's customer base.
Leading vendors like Sartorious and WuXi AppTec offer one-stop solutions spanning discovery, preclinical and manufacturing support for viral clearance. This single-source strategy helps them bag long-term contracts from big pharma and minimize customer attrition.
Segmental Analysis of Viral Clearance Market
Insights, By Type of Scale of Operation: Increasing R&D Investments is a Major Driver
In terms of by type of scale of operation, discovery phase sub-segment contributes the highest share of 41.7% in the market owing to increasing R&D investments.
The discovery phase of the viral clearance market contributes the highest share due to the significant investments made by pharmaceutical companies in research and development. During this initial stage of drug development, companies invest enormous resources into identifying and testing potential drug candidates. This involves screening large libraries of chemical and biological substances to detect any compounds exhibiting therapeutic effects against diseases.
Once candidates are found, lengthy investigations are carried out to understand the underlying mechanisms of action and interactions within the body. Cell assays, animal studies, and other preclinical experiments are extensively utilized to firmly establish safety and efficacy profiles before moving to human trials. Considering the high risks of failure at this stage, leading to wasted time and resources, pharmaceutical firms leave no stone unturned to maximize the chances of success.
Advancements in fields such as molecular biology, genetic engineering and computational science have enabled researchers to explore an exponentially wider range of possibilities compared to before. This has significantly boosted discovery efforts. At the same time, stringent regulatory norms along with increasing development costs are compelling companies to commit even greater funds in early research to reduce uncertainties later on.
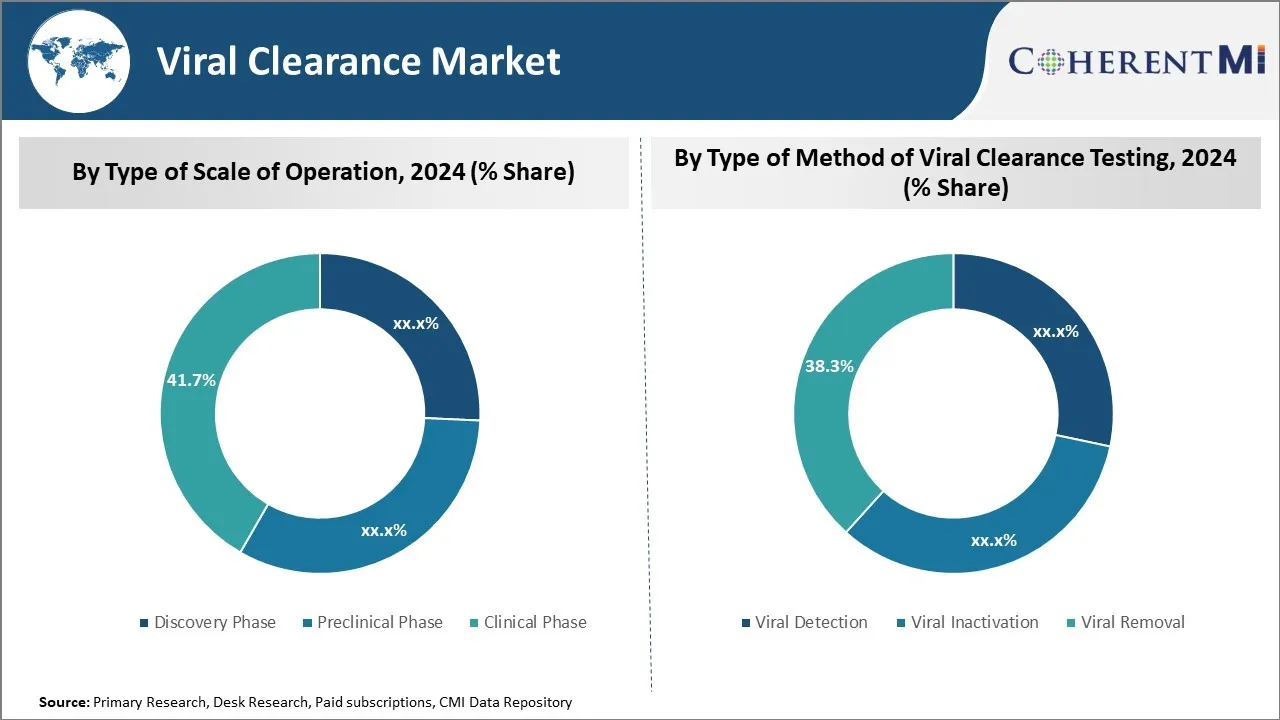
Insights, By Type of Method of Viral Clearance Testing: Viral Detection Sub-segment Had Critical Role in Ensuring Safety
Viral detection sub-segment contributes the highest share of 38.3% in the market owing to its critical role in ensuring safety. This is because detecting the presence or absence of viruses is the most fundamental step to ensure product safety and quality. All biological drugs, tissues, stem cell therapies and other materials must undergo sensitive detection assays to rule out potential viral contaminations before they can be approved for clinical use or commercial production.
Even trace amounts of infectious agents could prove harmful, especially for vulnerable patient groups like cancer patients or recipients of transplant organs. Regulators worldwide mandate developers to carry out robust viral screening using the most stringent assays. Any positive readings would mean the entire batch is unfit for use and leads to huge financial losses.
Additionally, as gene and cell-based therapies become more advanced, the risk of contaminated inputs transmitting new unknown viruses also grows. This demands continuous improvements to make tests more powerful and sophisticated.
Leading the detection segment are polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) methods due to their high sensitivity. Ongoing progress in sequencing technologies, pathway analyses and molecular probes is further enhancing capabilities to find even latent and uncultivable viruses. As biologics grow in use across diseases and new viral threats emerge, detection solutions enabling complete clearance validation assume utmost importance. Their critical role in protecting public health will sustain this segment's dominance of the viral clearance testing market.
Additional Insights of Viral Clearance Market
- Viral clearance testing is a critical step in the biopharmaceutical manufacturing process to ensure product safety and regulatory compliance. The increasing number of biologics and biopharmaceuticals in development has driven the demand for these services. The market is characterized by a mix of large, mid-sized, and small companies offering specialized services. Outsourcing has become a preferred option for biopharmaceutical manufacturers due to the cost and complexity of in-house testing.
Competitive overview of Viral Clearance Market
The major players operating in the Viral Clearance Market include Charles River Laboratories, Creative Biolabs, Eurofins Scientific, Microbac Laboratories, MilliporeSigma, Nelson Labs, Syngene International, Texcell, Vironova, and WuXi Biologics.
Viral Clearance Market Leaders
- Charles River Laboratories
- Creative Biolabs
- Eurofins Scientific
- Microbac Laboratories
- MilliporeSigma
Viral Clearance Market - Competitive Rivalry, 2024

Viral Clearance Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Viral Clearance Market
- In June 2023, Cytiva and BioCentriq received USD 15.8 million from NIIMBL to develop a viral and exotoxin clearance platform.
- In June 2023, Texcell opened a testing facility in North America to improve viral safety of biotherapeutics.
- In June 2023, Valo Therapeutics partnered with Texcell to study immune responses in Phase 1 clinical trial.
Viral Clearance Market Segmentation
- By Type of Scale of Operation
- Discovery Phase
- Preclinical Phase
- Clinical Phase
- By Type of Method of Viral Clearance Testing
- Viral Detection
- Viral Inactivation
- Viral Removal

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
What are the key factors hampering the growth of the Viral Clearance Market?
The high costs associated with viral clearance testing and stringent regulatory guidelines and requirement for specialized technical skills and expertise are the major factors hampering the growth of the Viral Clearance Market.
What are the major factors driving the Viral Clearance Market growth?
The increasing demand for viral clearance testing due to the rise in biologics and biopharmaceuticals and advancements in viral clearance technologies such as next-generation sequencing are the major factors driving the Viral Clearance Market.
Which is the leading Type Of Scale Of Operation in the Viral Clearance Market?
The leading Type of Scale of Operation segment is Clinical Phase.
Which are the major players operating in the Viral Clearance Market?
Charles River Laboratories, Creative Biolabs, Eurofins Scientific, Microbac Laboratories, MilliporeSigma, Nelson Labs, Syngene International, Texcell, Vironova, and WuXi Biologics are the major players.
What will be the CAGR of the Viral Clearance Market?
The CAGR of the Viral Clearance Market is projected to be 10% from 2024-2031.