Acute On Chronic Liver Failure (ACLF) Market Size - Analysis
The Acute on Chronic Liver Failure Market is witnessing positive trends which are expected to drive growth during the forecast period. There is an increased adoption of novel diagnostic tools and emergence of combination therapies for improved treatment outcomes of ACLF patients. Furthermore, the presence of strong product pipeline with drugs in late stages of clinical trials and growing research funding & investments for development of innovative treatment options for ACLF will further support the market growth in the coming years.
Market Size in USD Bn
CAGR5.5%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 5.5% |
| Market Concentration | High |
| Major Players | Promethera Biosciences, Beijing Continent Pharmaceutical Co, Ltd, Cellaion SA, Versantis, Novartis and Among Others |
please let us know !
Acute On Chronic Liver Failure (ACLF) Market Trends
With changing lifestyle and diet patterns across the world, prevalence of chronic liver diseases witnessed substantial increase over last few decades. The cases of liver cirrhosis are rising in the urban areas due to the rise in consumption of alcohol, lack of physical activities and sedentary lifestyle. According to WHO, in 2023 nearly 55 million people were living with chronic viral hepatitis infection worldwide which is a major risk factor for development of cirrhosis in long term. Once cirrhosis is established, it further progresses to end stage liver disease commonly called as decompensated cirrhosis which requires liver transplantation. Decompensated cirrhosis is a major predisposing condition for ACLF.
Despite advancements in critical care, present medical management of ACLF remains largely supportive without definite therapies. Solid organ transplant being the only established curative option has massive restrictions owing to scarcity of organs and procedural challenges. This major unmet need has resulted in active research initiatives exploring novel cell-based and gene therapy approaches for ACLF.
With improvements in vector engineering and cell processing techniques, gene and cell-based therapies hold immense promise as disease modifying approaches for ACLF management. If ongoing rigorous research efforts validate their safety, efficacy and commercial viability, they may revolutionize treatment landscape of this severe emerging disease. This would provide enormous scope for companies engaged in developing such cutting-edge regenerative medicine platforms to target Acute on Chronic Liver Failure Market in future. Novel biological treatment strategies are certainly needed to lower alarming mortality and transplant requirement in ACLF and cell and gene-based therapies may offer substantive solutions.
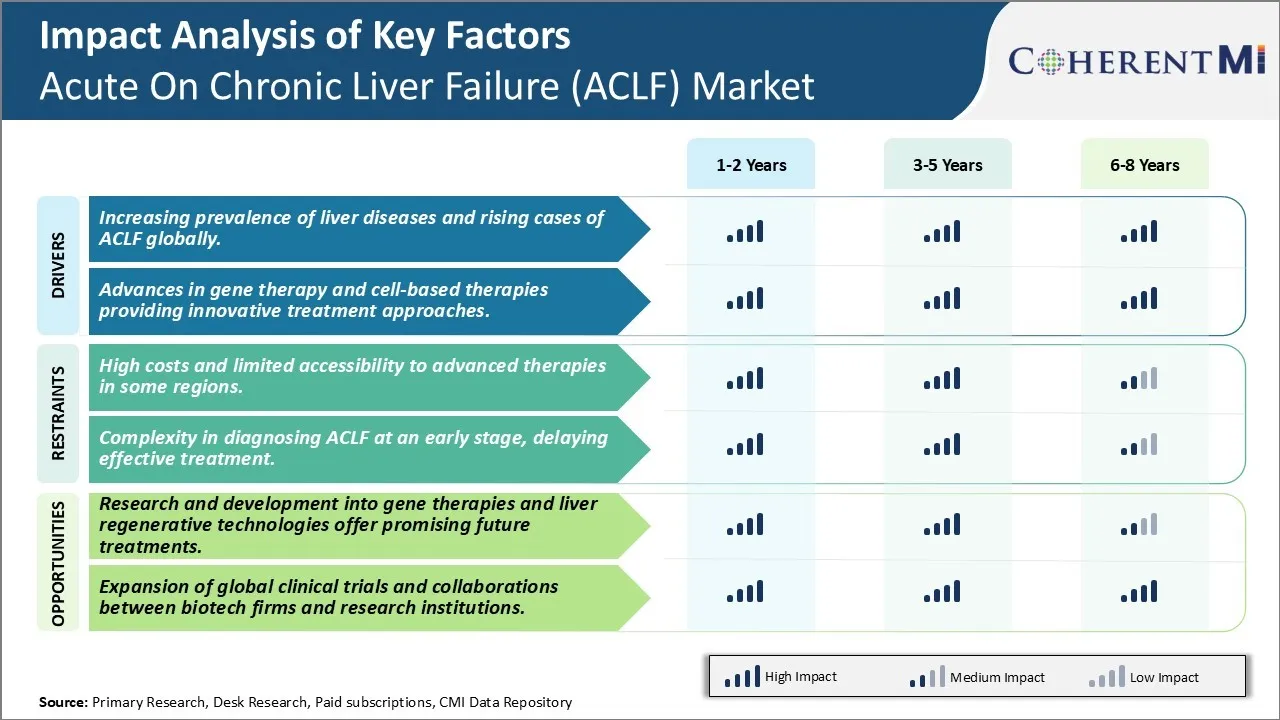
One of the major challenges faced by the Acute on Chronic Liver Failure (ACLF) market is the high costs and limited accessibility to advanced therapies in some regions of the world. ACLF is a disease that requires timely diagnosis and treatment to increase chances of survival. However, the therapies available for treating ACLF such as liver transplantation and cell-based therapies are extremely expensive, making it difficult for large sections of patients to access them. Liver transplantation, which offers the best outcome for advanced stage ACLF patients, costs anywhere between USD 200,000 to USD 300,000 in the US alone. Similarly, novel cell therapies involving use of mesenchymal stem cells are also priced very high at around USD50,000 per patient. While such advanced treatments have started to show good results, their exorbitant price tags place them out of reach for most patients globally, particularly in developing nations and underserved areas where the incidence of liver disease is quite high. This establishes cost as a major limiting factor for the growth of Acute on Chronic Liver Failure Market since it prevents new as well as optimized therapies from reaching all patients in need. Unless measures are taken to make therapies more affordable, a large population will continue to remain untapped, adversely impacting the commercial market potential.
Market Opportunity: Research and Development in Gene Therapies and Liver Regenerative Technologies Offer Promising Future Treatments.
Prescribers preferences of Acute On Chronic Liver Failure (ACLF) Market
ACLF is characterized by acute deterioration of pre-existing chronic liver disease. It is classified into 3 grades based on severity of organ failure. For grade 1 ACLF (mild), the preferred first-line treatment includes medications to lower serum bilirubin such as ursodeoxycholic acid (Ursofalk).
In grade 3 ACLF (severe), the focus shifts to organ support through critical care measures. Prescribers commonly prefer broad spectrum antibiotics to prevent sepsis, for example meropenem (Meronem) along with vasopressors/inotropes to support circulation. They also administer proton pump inhibitors like pantoprazole (Pantocid) to reduce risk of gastrointestinal bleeding.
Treatment Option Analysis of Acute On Chronic Liver Failure (ACLF) Market
ACLF is classified into 3 stages - A, B, C based on disease severity. Stage A involves mild hepatic encephalopathy and minimal organ dysfunction. Stage B shows recurrent or persistent hepatic encephalopathy with additional organ dysfunction. Stage C displays multiple organ failures.
In Stage B, along with the first-line medications, lactulose is prescribed to reduce elevated ammonia levels causing hepatic encephalopathy. Infections are aggressively treated with broad spectrum antibiotics like Piperacillin-Tazobactam. Branched-chain amino acids like Levocarnitine aid in nitrogen removal.
The treatments aim to suppress complications, reverse precipitating factors, provide organ function support and prevent progression at each stage. Early-stage patients have better outcomes with optimized medical management while late-stage management focuses on bridging patients to transplant.
Key winning strategies adopted by key players of Acute On Chronic Liver Failure (ACLF) Market
Product Innovation: One of the most important strategies adopted by pharmaceutical companies to gain an edge in the Acute on Chronic Liver Failure Market has been continuous product innovation through extensive R&D efforts. For instance, Terlipressin by Mallinckrodt helps manage vascular complications and improves liver function. This product innovation helped Mallinckrodt gain a first-mover advantage and significantly increased its market share.
Focus on Emerging Markets: Given the rising prevalence of ACLF in developing nations, key players have focused their commercialization efforts on emerging markets which offer higher growth opportunities.
Segmental Analysis of Acute On Chronic Liver Failure (ACLF) Market
-market-by-type-of-treatment.webp)
Insights, By Type of Treatment, Pharmacological Contributes Highest Market Share Based on Demand for Targeted Drug Therapies.
Therapies that reduce inflammation and restore liver function are seeing particularly high demand. Biologics that neutralize cytokines like TNF-α and IL-6 have shown promise in alleviating systemic inflammation. Several new molecular entities are also under development that can activate hepatocyte regeneration pathways, inhibit fibrogenesis, and induce diuretic effects. With pharmacological options demonstrating better clinical outcomes than supportive care alone, their usage is becoming more widespread. This is a key factor driving the growth of this segment.
-market-by-end-user.webp) Insights, By End-user, Hospitals Segment to Register a Remarkable Market Share in the Forecast Period.
Insights, By End-user, Hospitals Segment to Register a Remarkable Market Share in the Forecast Period.
Hospitals are equipped to offer the intensive care, advanced diagnostics, and round-the-clock supervision that ACLF patients need. They have critical care units with facilities for mechanical ventilation, renal replacement therapy, and circulatory support. Dedicated liver transplantation centers also aid in managing end-stage cases. The availability of hepatologists, pulmonologists, nephrologists, and other personnel under one roof streamlines complex care. This specialized multi-disciplinary hospital environment addresses the holistic needs of the ACLF patient, contributing significantly to their share of the market.
Additional Insights of Acute On Chronic Liver Failure (ACLF) Market
ACLF is a life-threatening condition that significantly increases the risk of multi-organ failure and mortality. Early diagnosis and management are critical to improving outcomes, but the high cost of treatment and the complexity of liver regeneration remain major challenges. Innovations such as Promethera Biosciences' HepaStem, a promising cell-based therapy, are leading the way in liver regeneration. HepaStem aims to restore liver function and prevent further organ damage by modulating inflammation and promoting hepatic recovery. Similarly, Beijing Continent Pharmaceutical Co, Ltd's F573 shows promise in targeting caspases, enzymes involved in inflammation and cell death, offering a new therapeutic approach for reducing the complications associated with ACLF. The development of these novel therapies could revolutionize ACLF treatment, especially as more clinical trials are conducted and partnerships are formed between research institutions and biotechnology companies.
Regional Insights:
Competitive overview of Acute On Chronic Liver Failure (ACLF) Market
The major players operating in the Acute On Chronic Liver Failure (ACLF) Market include Promethera Biosciences, Beijing Continent Pharmaceutical Co, Ltd, Cellaion SA, Versantis, Novartis, Zydus Lifesciences, Grifols Therapeutics, GENFIT, RHEACELL, Martin Pharmaceuticals and Cipla.
Acute On Chronic Liver Failure (ACLF) Market Leaders
- Promethera Biosciences
- Beijing Continent Pharmaceutical Co, Ltd
- Cellaion SA
- Versantis
- Novartis
Acute On Chronic Liver Failure (ACLF) Market - Competitive Rivalry

Acute On Chronic Liver Failure (ACLF) Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Acute On Chronic Liver Failure (ACLF) Market
- In May 2024, Promethera Biosciences reported that HepaStem, a cell-based therapy, has shown promise in improving liver regeneration and reducing inflammation in patients with ACLF during its Phase II trial.
- In March 2024, Beijing Continent Pharmaceutical Co, Ltd advanced its F573 drug in Phase II clinical trials, highlighting its efficacy in reducing liver failure-related complications through the inhibition of caspases.
- HepaStem, developed by Promethera Biosciences, is a leading cell-based therapy currently in Phase II clinical trials. It aims to regenerate liver function by reducing inflammation and supporting organ recovery.
- F573, by Beijing Continent Pharmaceutical Co, Ltd, focuses on inhibiting cell death and inflammation through its caspase-targeting mechanism, providing a novel approach to treating ACLF.
Acute On Chronic Liver Failure (ACLF) Market Segmentation
- By Type of Treatment
- Pharmacological
- Supportive Care
- By End-user
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics

Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Frequently Asked Questions :
How Big is the Acute On Chronic Liver Failure (ACLF) Market?
The Global Acute On Chronic Liver Failure (ACLF) Market is estimated to be valued at USD 4.43 billion in 2025 and is expected to reach USD 6.44 billion by 2032.
What will be the CAGR of the Acute On Chronic Liver Failure (ACLF) Market?
The CAGR of the Acute On Chronic Liver Failure (ACLF) Market is projected to be 5.3% from 2024 to 2031.
What are the major factors driving the Acute On Chronic Liver Failure (ACLF) Market growth?
The increasing prevalence of liver diseases and rising cases of ACLF globally and advances in gene therapy and cell-based therapies providing innovative treatment approaches are the major factors driving the Acute On Chronic Liver Failure (ACLF) Market.
What are the key factors hampering the growth of the Acute On Chronic Liver Failure (ACLF) Market?
The high costs and limited accessibility to advanced therapies in some regions and complexity in diagnosing ACLF at an early stage, delaying effective treatment are the major factor hampering the growth of the Acute On Chronic Liver Failure (ACLF) Market.
Which is the leading Type of Treatment in the Acute On Chronic Liver Failure (ACLF) Market?
Pharmacological is the leading Type of Treatment segment.
Which are the major players operating in the Acute On Chronic Liver Failure (ACLF) Market?
Promethera Biosciences, Beijing Continent Pharmaceutical Co, Ltd, Cellaion SA, Versantis, Novartis, Zydus Lifesciences, Grifols Therapeutics, GENFIT, RHEACELL, Martin Pharmaceuticals, Cipla are the major players.