Alport Syndrome Market Size - Analysis
The market is witnessing positive trends with the development and approval of novel drug candidates to treat Alport syndrome. Key players are focused on developing therapies that can slow kidney disease progression and prevent hearing and vision loss in patients. Furthermore, improving reimbursement landscape for orphan drugs in major markets is encouraging pharmaceutical companies to advance their drug pipelines for Alport syndrome, which will drive market revenues over the forecast period.
Market Size in USD Bn
CAGR6.9%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 6.9% |
| Market Concentration | High |
| Major Players | Eloxx Pharmaceuticals, Chinook Therapeutics, Travere Therapeutics, Reata Pharmaceuticals, Bayer and Among Others |
please let us know !
Alport Syndrome Market Trends
It is estimated that the overall prevalence of Alport syndrome is increasing globally, especially for the X-linked subtype which accounts for around 85% of total cases. As per genetics and inheritance pattern, the X-linked form of the disease is more common in males as the defective gene is located on the X chromosome. Females have a lower risk of getting the disease as they have a second normal X chromosome that provides a backup copy of the gene. However, recent research has found that prevalence rates may be higher than previously reported and epidemiological data also suggests increasing diagnosis rates in the last decade.
There is significant research activity currently going on to develop novel therapeutic options targeting the underlying genetic cause of Alport syndrome. A majority of the drug candidates in development aim to address the specific genetic mutations through gene silencing, gene editing or gene therapy approaches. Several biopharma companies have identified gene targets and are exploring antisense oligonucleotides, siRNA, CRISPR and other gene modifying technologies. A few therapies have also reached clinical trials and initial safety and efficacy results have been promising.
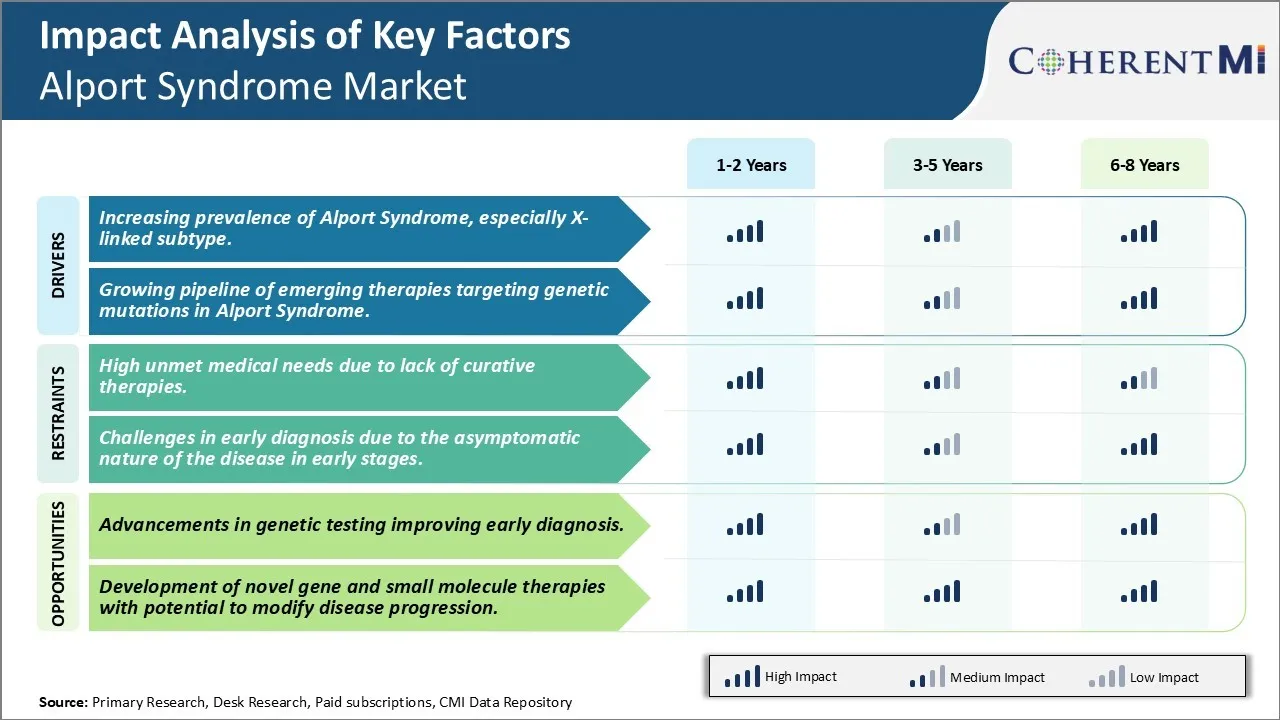
Market Challenge - High Unmet Medical Needs Due to Lack of Curative Therapies.
Improvements in genetic testing technologies are helping enhance early diagnosis of Alport syndrome. Next-generation sequencing techniques are making it possible to quickly and accurately identify mutations in COL4A3/4/5 genes that are responsible for causing the condition. There is an increased ability to confirm a diagnosis, even in patients with few or no symptoms yet. Earlier diagnosis allows for timely management through early interventions aimed at reducing risks of end-stage renal disease and other complications. It also helps provide families with proper guidance on reproductive risks and screening options for other family members. As genetic testing becomes more widely used primary care, more patients are likely to receive a confirmed diagnosis at an earlier stage of the disease. This growing ability to detect Alport syndrome sooner presents opportunities for drug developers to target patients earlier in their treatment journey.
Prescribers preferences of Alport Syndrome Market
Alport Syndrome is a rare genetic disorder that affects the kidney, cochlea of the inner ear, and eyes. Treatment is focused on slowing kidney disease progression and managing symptoms.
As kidney function declines, patients transition to Stage 3 chronic kidney disease, requiring additional medications. Prescribers commonly add potassium binders like Kayexalate to control potassium levels. They may also prescribe phosphate binders such as Calcium Carbonate brands like Tums or Oscal to lower serum phosphorus in preparation for dialysis.
Once Stage 5 renal failure is reached, long-term dialysis is needed. Most prescribers initiate hemodialysis using Cimino fistulas surgically placed in the forearm. Peritoneal dialysis using brands like Dianeal is an alternative but less common due to peritoneal complications in Alport patients. Prescribers closely monitor dialysis patients on anticoagulants like Warfarin. Kidney transplantation may be considered for eligible patients. The child's gender, age of onset, and rate of progression impact treatability and long-term outcomes. Familial mutations also affect prescribers' recommendation of aggressive therapies.
Treatment Option Analysis of Alport Syndrome Market
Alport syndrome has progressive stages depending on loss of kidney function. Initial treatment focuses on slowing progression by targeting issues like high blood pressure and proteinuria. Later stages involve dialysis and transplantation.
As glomerular filtration declines, randomized trials show inhibitors reduce loss of function by 30-40% versus other antihypertensives. Their dual action lowering both blood pressure and proteinuria make them ideal for delaying disease progression at this stage.
In summary, ACE inhibitors/ARBs are the first-line treatment throughout Alport syndrome due to compelling evidence they slow progression by targeted mechanisms. Dialysis and transplantation become necessary later as options to replace lost renal function.
Key winning strategies adopted by key players of Alport Syndrome Market
Focus on expanding treatment options: Leading pharma companies are investing heavily in R&D to develop new and improved treatment options for Alport syndrome. This allows them to address unmet needs and expand their market share. For example, Regulus Therapeutics developed RG-012, an oligonucleotide therapy targeting the underlying cause of Alport syndrome. This is the first therapy in clinical trials aimed at halting the progression of the disease.
Acquisitions of Niche Players: Large pharma companies are acquiring smaller biotechs working on Alport syndrome to gain access to their candidate pipeline and expertise. For instance, in 2021 Takeda acquired Lynke Therapeutics to obtain LYN-006, an antibody therapy in Phase 1 trials for Alport syndrome and other forms of chronic kidney disease. This strengthened Takeda's renal portfolio.
These strategic initiatives by leaders such as Reata, Regulus and Takeda have helped significantly expand therapeutic options for Alport syndrome patients over the past decade.
Segmental Analysis of Alport Syndrome Market
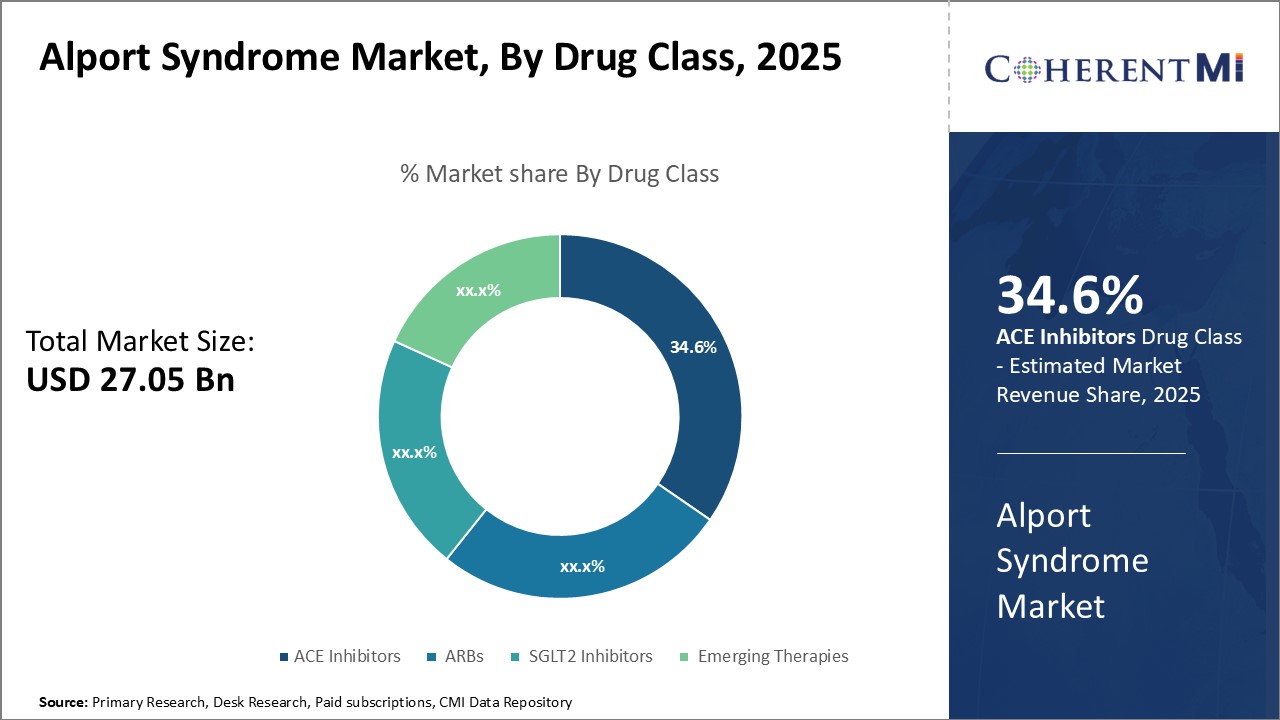 Insights, By Drug Class, Clinical Efficacy Drives Market Dominance of ACE inhibitors
Insights, By Drug Class, Clinical Efficacy Drives Market Dominance of ACE inhibitorsBy Drug Class, ACE inhibitors is expected to contribute the highest share 34.6% in 2025 owing to its established clinical efficacy in managing hypertension and delaying progression of kidney disease in Alport syndrome patients. ACE inhibitors are recommended as first-line therapy to control blood pressure, as uncontrolled hypertension can accelerate renal function decline. Long-term usage data has proven that ACE inhibitors effectively lower blood pressure and proteinuria levels in Alport syndrome patients. This clinical evidence provides doctors and patients greater confidence in opting for ACE inhibitors over other classes. Additionally, several generic versions are now available, making ACE inhibitors more affordable for long-term therapy compared to newly emerging drug classes. The extensive clinical experience and efficacy database have solidified ACE inhibitors as the standard of care in current treatment guidelines, firmly securing its leading position in the Alport syndrome drug market.
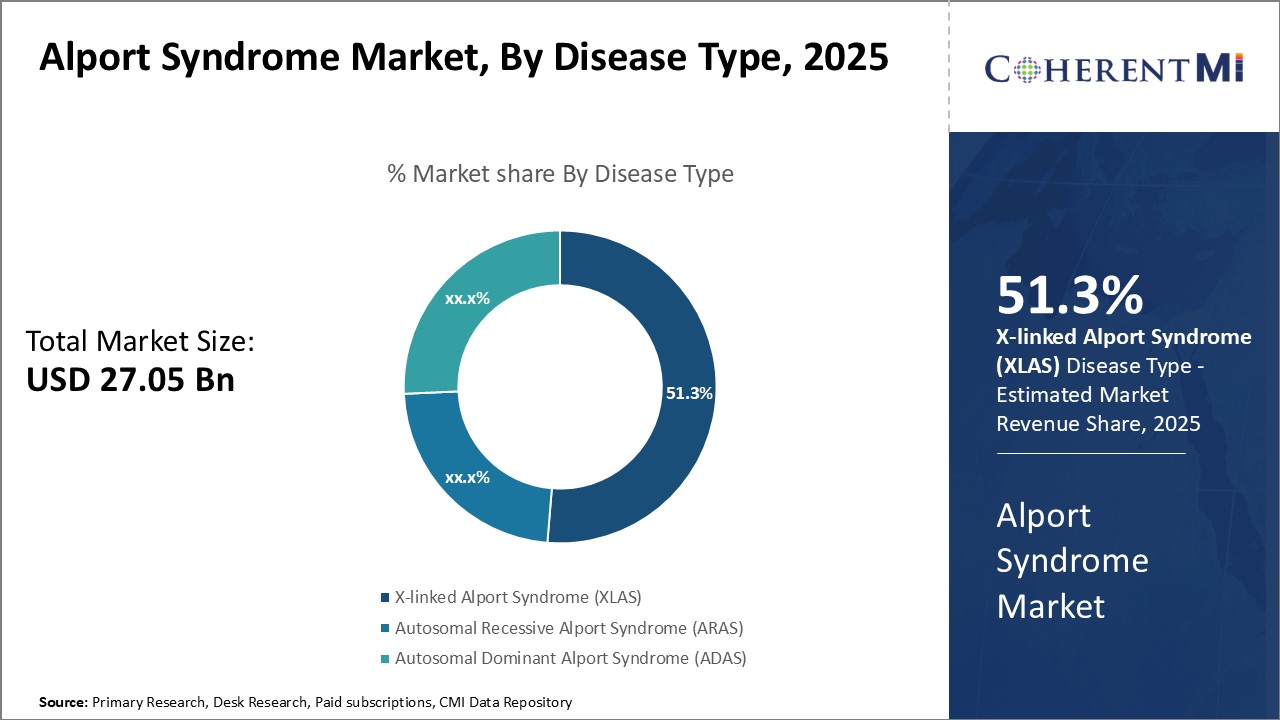
By Disease Type, X-linked Alport Syndrome (XLAS) is expected to contribute the highest share 51.3% in 2025 owing to its high incidence rate. XLAS accounts for around 80% of all Alport syndrome cases. Being an X-linked genetic disorder, it occurs more frequently in males than females. Studies have reported the incidence rate of XLAS to be 1 in 5,000 among males. In comparison, the incidence rates of autosomal recessive and dominant variants are much lower, estimated to be 1 in 50,000 for ARAS and 1 in 100,000 for ADAS. The sheer volume of XLAS patients due to its relatively high incidence rate makes it the leading disease type segment in this patient population. Additionally, loss of renal function tends to occur earlier in life for XLAS patients compared to other variants. This places a bigger disease management burden for the XLAS segment.
Insights, By Patient Type, Higher Disease Severity Drives Adult Patient Segment Dominance.
Additional Insights of Alport Syndrome Market
Alport Syndrome is a rare genetic disorder affecting kidneys, hearing, and eyes, primarily due to mutations in type IV collagen. Most patients are undiagnosed due to the disease's asymptomatic progression. The market is driven by the urgent need for new treatments as current therapies only delay disease progression without providing a cure. Emerging gene therapies like ELX-02 and other novel drugs such as Atrasentan hold promise in addressing this gap. The US accounts for the highest market share due to a larger patient pool and better access to advanced therapies. Future market growth is expected with advancements in genetic testing and the introduction of new treatments that could modify the disease course.
Competitive overview of Alport Syndrome Market
The major players operating in the Alport Syndrome Market include Eloxx Pharmaceuticals, Chinook Therapeutics, Travere Therapeutics, Reata Pharmaceuticals, Bayer, River 3 Renal Corp, Calliditas Therapeutics, Novartis, Evotec, Genzyme Corp, TMC Pharma Services Ltd and Shanghai Children's Hospital.
Alport Syndrome Market Leaders
- Eloxx Pharmaceuticals
- Chinook Therapeutics
- Travere Therapeutics
- Reata Pharmaceuticals
- Bayer
Alport Syndrome Market - Competitive Rivalry

Alport Syndrome Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Alport Syndrome Market
- September 2023: Eloxx Pharmaceuticals advanced its gene therapy ELX-02 to Phase II clinical development, aiming to target nonsense mutations in Alport Syndrome patients.
- July 2023: Chinook Therapeutics initiated a Phase II trial for Atrasentan, an ETA receptor antagonist, which aims to reduce proteinuria in Alport Syndrome and other kidney diseases.
Alport Syndrome Market Segmentation
- By Drug Class
- ACE Inhibitors
- ARBs
- SGLT2 Inhibitors
- Emerging Therapies
- By Disease Type
- X-linked Alport Syndrome (XLAS): 51.3%
- Autosomal Recessive Alport Syndrome (ARAS): 30.7%
- Autosomal Dominant Alport Syndrome (ADAS): 18.0%
- By Patient Type
- Adult Patients
- Pediatric Patients

Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Frequently Asked Questions :
How Big is the Alport Syndrome Market?
The Global Alport Syndrome Market is estimated to be valued at USD 27.05 Bn in 2025 and is expected to reach USD 43.15 Bn by 2032.
What will be the CAGR of the Alport Syndrome Market?
The CAGR of the Alport Syndrome Market is projected to be 6.7% from 2024-2031.
What are the major factors driving the Alport Syndrome Market growth?
The increasing prevalence of Alport syndrome, especially x-linked subtype and growing pipeline of emerging therapies targeting genetic mutations in Alport syndrome are the major factor driving the Alport Syndrome Market.
What are the key factors hampering the growth of the Alport Syndrome Market?
The high unmet medical needs due to lack of curative therapies and challenges in early diagnosis due to the asymptomatic nature of the disease in early stages are the major factor hampering the growth of the Alport Syndrome Market.
Which is the leading Drug Class in the Alport Syndrome Market?
ACE Inhibitors is the leading drug class segment.
Which are the major players operating in the Alport Syndrome Market?
Eloxx Pharmaceuticals, Chinook Therapeutics, Travere Therapeutics, Reata Pharmaceuticals, Bayer, River 3 Renal Corp, Calliditas Therapeutics, Novartis, Evotec, Genzyme Corp, TMC Pharma Services Ltd, Shanghai Children's Hospital are the major players.