Bacterial Vaginosis Market Size - Analysis
The market has been witnessing positive trends over the past few years. Factors such as rising prevalence of bacterial vaginosis infections and growth in female population have been driving the adoption of bacterial vaginosis treatment and diagnostics.
Market Size in USD Bn
CAGR8.9%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 8.9% |
| Market Concentration | Medium |
| Major Players | Symbiomix Therapeutics (Lupin Pharmaceuticals), Bayer AG, Pfizer Inc., Sanofi S.A., Teva Pharmaceutical Industries and Among Others |
please let us know !
Bacterial Vaginosis Market Trends
Bacterial vaginosis or BV is one of the most common causes of vaginal infections amongst women. As per our research, over the last few years the worldwide prevalence of BV cases has shown a notable rise. BV often causes unpleasant symptoms such as abnormal vaginal discharge and fishy odor which impacts the quality of life and well-being of affected women. This has led to a growing demand for effective therapies that can alleviate discomfort faster and cure the infection completely.
The rising global disease prevalence consequently spurs the total market demand for reliable BV therapies. Pharma firms actively work on new drug formulations, combination therapies and diagnostic tools to address unmet needs and expand their market share. Overall modern medicine appears to be benefitting the most from this upward trend in infections caused by bacterial vaginosis worldwide.
Market Driver - Enhanced Public Health Campaigns are Raising Awareness About Women's Reproductive Health
These campaigns highlight how frequent vaginal infections can disrupt the natural pH balance in the genital tract making women more susceptible to contracting sexually transmitted diseases. They emphasize that BV, though a mild condition by itself should not be ignored, as it could potentially enable opportunistic infections to take hold.
A result of these sustained drives has been a surge in health-seeking behavior. More women now visit general physicians or gynecologists on noticing any unusual vaginal discharge. Overall, such cultivation of knowledge aids both individual well-being and public health by curbing complications at mass scale.
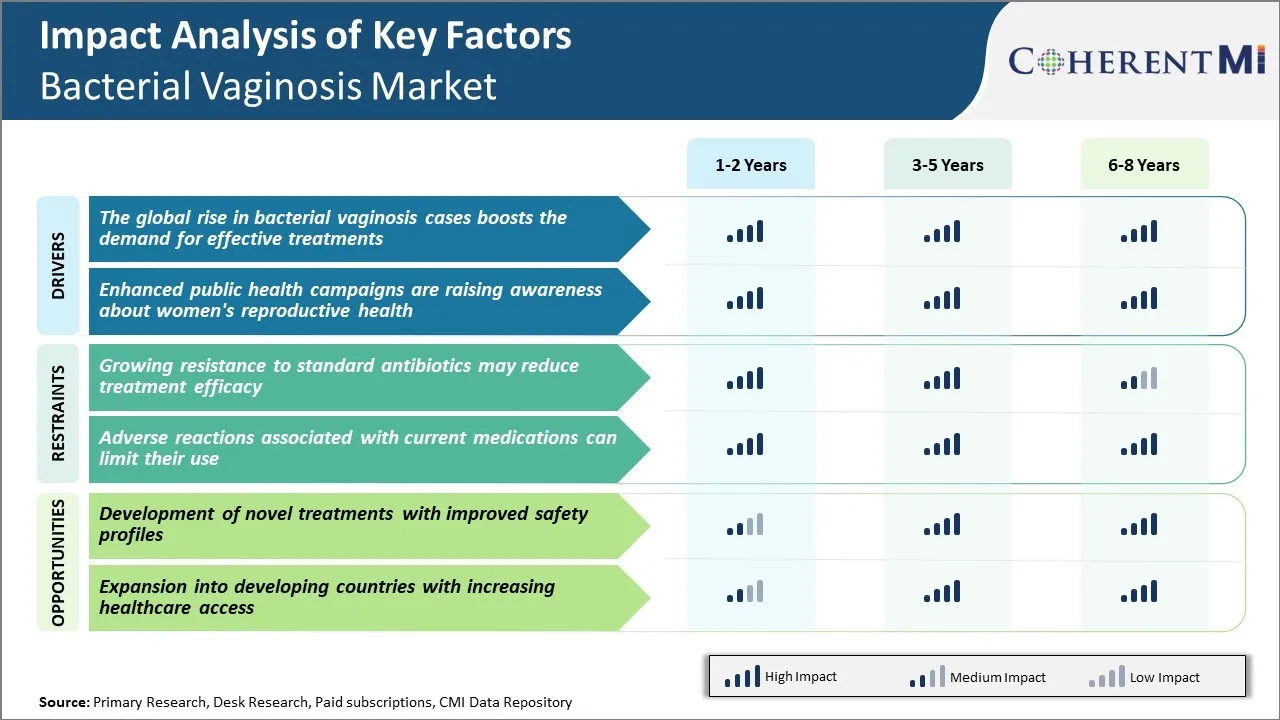
One of the major challenges facing the bacterial vaginosis market is the growing resistance to standard antibiotic treatments being used currently. Bacterial vaginosis is commonly treated with antibiotics like metronidazole and clindamycin.
With limited treatment options available, antibiotic resistance can potentially worsen patient outcomes and may lead to more frequent relapses or recurrent infections. It could also drive-up healthcare costs if patients need longer or more complex or expensive therapy options. This growing resistance further highlights the need for novel treatment alternatives with new mechanisms of action to circumvent emerging resistance issues and provide more effective solutions for BV management over the long term.
Market Opportunity - Development of Novel Treatments with Improved Safety Profiles
Also, recent studies have indicated potential long-term side effects with prolonged antibiotic usage including increased risks of antibiotic resistance, allergy and opportunistic infections. This presents a large unmet need for safer and better-tolerated non-antibiotic treatment alternatives.
Prescribers preferences of Bacterial Vaginosis Market
Bacterial Vaginosis (BV) is commonly treated via a two-line approach. For mild cases, doctors typically prescribe a short course of topical therapy with anti-bacterials like clindamycin vaginal cream (Clindesse). This line of treatment provides relief from symptoms like discharge and odor within a week.
The stage and recurrence rate of the infection also guides prescribers. Chronic or recurrent BV may signal underlying issues, prompting the use of combination therapy like metronidazole with vaginal probiotics or estrogen. Cost, ease of administration, and insurance coverage further impact drug selection at each line of treatment. This ensures a balance between efficacy, safety, adherence, and affordability for the patient.
Treatment Option Analysis of Bacterial Vaginosis Market
Bacterial Vaginosis has three main stages - mild, moderate, and severe - determined by symptoms and test results. For mild cases, over-the-counter treatment options include clindamycin creams applied intravaginally for 5-7 days. Brand names include Clindagel and Clindesse. This is a good first-line option due to ease of use.
If the initial antibiotic treatment does not work, further testing is required to rule out other infections or to determine if the identified organism has developed resistance. Second-line options depend on risk factors and culture/sensitivity results but may include 10–14-day courses of newer antibiotics like tinidazole (Tindamax) or longer-term suppressive therapy using acidifying gels like Balance Activ. Close monitoring of high-risk cases is also imperative to manage recurrences early.
Key winning strategies adopted by key players of Bacterial Vaginosis Market
Product innovation and expansion: Launching new and improved treatment solutions has helped companies gain an edge. For instance, in 2019, Bayer launched a new treatment option Clindamax CV, an expansion of its existing Clindamycin products for BV. The gel formulation ensures higher concentration of the drug in the vaginal tissues where it is needed.
Co-marketing agreements: Partnerships allow companies to leverage each other's core competencies and marketing networks. In 2016, Therapic signed an agreement with Meda Pharma to co-market and sell its prescription gel for BV, Solosec, across European markets. Within a year of launch, Solosec recorded €12 million in sales. This validated Therapic's marketing strategy of teaming up with an experienced partner in women's health.
Segmental Analysis of Bacterial Vaginosis Market
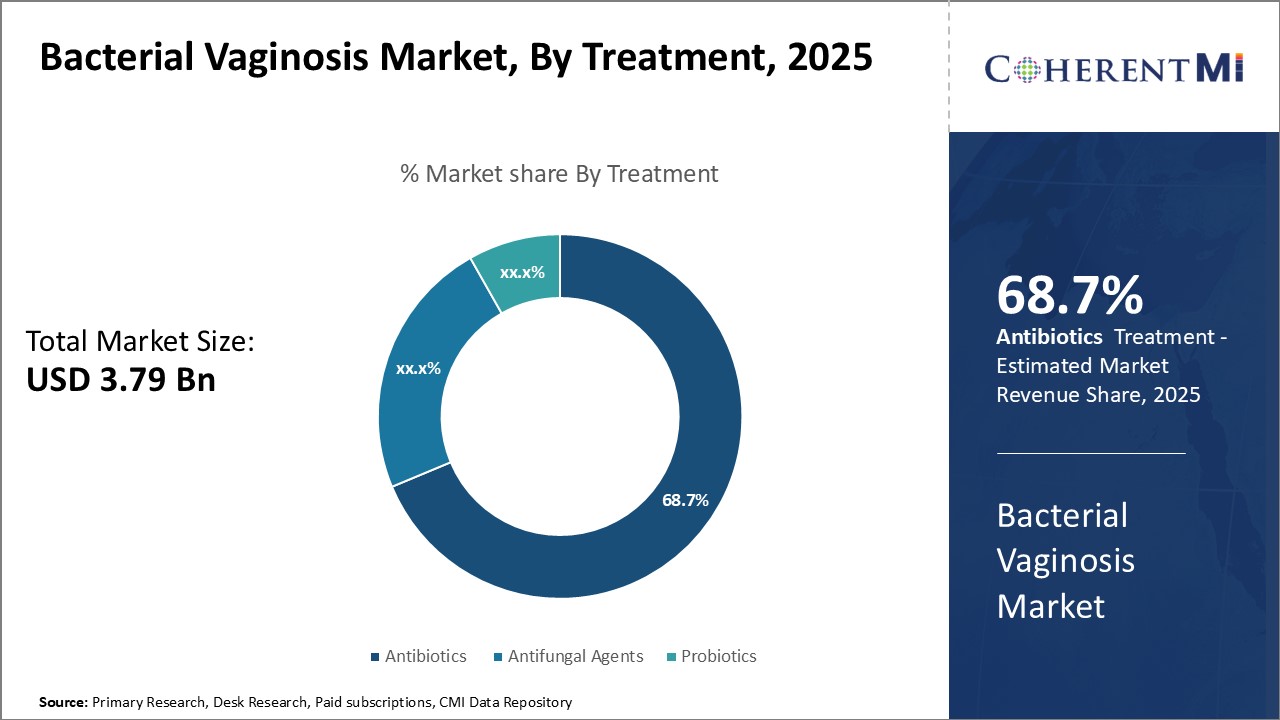 Insights, By Treatment: High Efficacy in Eradicating Causative Bacteria Drives Antibiotics Segment Dominance
Insights, By Treatment: High Efficacy in Eradicating Causative Bacteria Drives Antibiotics Segment DominanceIn terms of treatment, antibiotics contributes the highest share of the market owing to their high efficacy in eradicating the causative bacteria. Antibiotics such as metronidazole are considered the first line treatment for bacterial vaginosis due to their ability to kill anaerobic bacteria like Gardnerella vaginalis that cause the infection.
Other antibiotics used include clindamycin and tinidazole which also exhibit strong bactericidal activity against anaerobes. The immediate relief from symptoms provided by antibiotics further increases patient compliance with the treatment regimen. This leads to higher cure rates and reduces the risk of recurrence when compared to alternative treatment options.
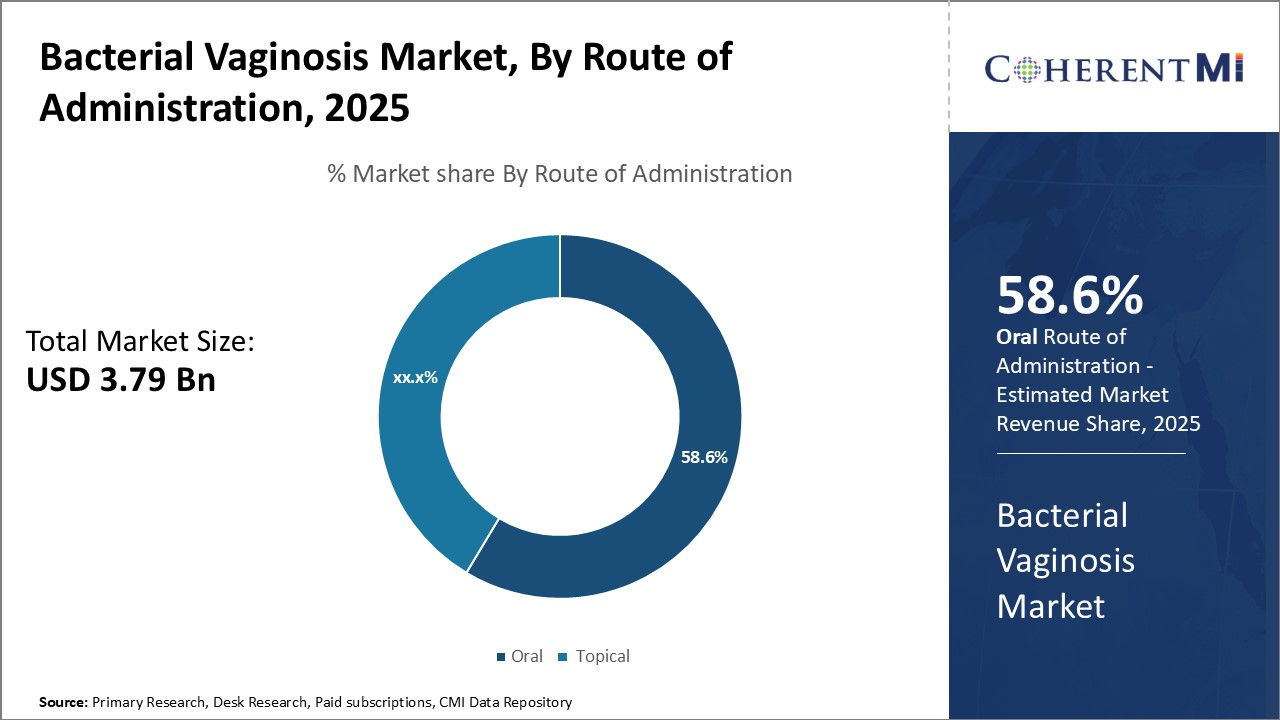
Insights, By Route of Administration: Oral administration Enhances Treatment Convenience and Adherence
The oral medications also do not require special applicators for insertion like topical formulations. This avoids any pain or mess associated with creams and gels. Further, oral medications have consistent and sustained release of the active ingredient, achieving therapeutic drug levels efficiently. Their simple once or twice daily dosing regimen fits well into patient's daily schedules. This boosts treatment adherence and compliance substantially.
Among the distribution channels in the bacterial vaginosis market, hospitals contribute the highest share owing to widespread accessibility and acceptance. As the primary point of care for most bacterial vaginosis patients, hospitals provide easy access to diagnosis and treatment options. Comprehensive gynecology services under one roof address all patient needs efficiently.
Further, hospitals enjoy high credibility and trustworthiness among patients accustomed to receiving specialist care in this setting. Established brands and positive past experiences drive repeat patronization of hospital-based treatment. The confidence in hospital expertise combined with accessibility and affordability has made this the preferred and most widely accepted channel for bacterial vaginosis management.
Additional Insights of Bacterial Vaginosis Market
- High Recurrence Rates: Studies indicate that up to 30% of women experience a recurrence of bacterial vaginosis within three months of treatment, highlighting the need for more effective therapies.
- Global Impact: Bacterial vaginosis affects an estimated 21 million women worldwide annually, making it the most common vaginal infection among women of reproductive age.
- Introduction of Single-Dose Therapies: The approval of single-dose oral treatments like Solosec has significantly improved patient adherence by simplifying dosing regimens.
- Collaborative Research Efforts: Partnerships between pharmaceutical companies and research institutions are focusing on understanding the microbiome's role in bacterial vaginosis, which may lead to more targeted therapies.
Competitive overview of Bacterial Vaginosis Market
The major players operating in the Bacterial Vaginosis Market include Symbiomix Therapeutics (Lupin Pharmaceuticals), Bayer AG, Pfizer Inc., Sanofi S.A., Teva Pharmaceutical Industries, Abbott Laboratories, Novartis AG, Johnson & Johnson, and Merck & Co., Inc.
Bacterial Vaginosis Market Leaders
- Symbiomix Therapeutics (Lupin Pharmaceuticals)
- Bayer AG
- Pfizer Inc.
- Sanofi S.A.
- Teva Pharmaceutical Industries
Bacterial Vaginosis Market - Competitive Rivalry

Bacterial Vaginosis Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Bacterial Vaginosis Market
- In June 2023, Symbiomix Therapeutics expanded the availability of Solosec to European markets. This move aims to enhance access to single-dose treatments, potentially improving patient compliance and outcomes. Symbiomix Therapeutics was acquired by Lupin Pharmaceuticals in 2017. While Lupin has a global presence, there were no official statements or regulatory approvals reported that confirm the expansion of Solosec into Europe by that date.
- In September 2023, Pfizer Inc. launched a clinical trial for a new probiotic therapy targeting bacterial vaginosis. The initiative could lead to alternative treatments that reduce reliance on antibiotics and address antibiotic resistance concerns.
Bacterial Vaginosis Market Segmentation
- By Treatment
- Antibiotics
- Metronidazole
- Clindamycin
- Tinidazole
- Antifungal Agents
- Probiotics
- Antibiotics
- By Route of Administration
- Oral
- Topical
- Creams
- Gels
- Suppositories
- By Distribution Channel
- Hospitals
- Clinics
- Retail Pharmacies
- Online Pharmacies

Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Frequently Asked Questions :
How big is the bacterial vaginosis market?
The bacterial vaginosis market is estimated to be valued at USD 3.79 billion in 2025 and is expected to reach USD 6.88 billion by 2032.
What are the key factors hampering the growth of the bacterial vaginosis market?
The growing resistance to standard antibiotics may reduce treatment efficacy and adverse reactions associated with current medications can limit their use are the major factors hampering the growth of the bacterial vaginosis market.
What are the major factors driving the bacterial vaginosis market growth?
The global rise in bacterial vaginosis cases boosts the demand for effective treatments and enhanced public health campaigns are raising awareness about women's reproductive health are the major factors driving the bacterial vaginosis market.
Which is the leading treatment in the bacterial vaginosis market?
The leading treatment segment is antibiotics.
Which are the major players operating in the bacterial vaginosis market?
Symbiomix Therapeutics (Lupin Pharmaceuticals), Bayer AG, Pfizer Inc., Sanofi S.A., Teva Pharmaceutical Industries, Abbott Laboratories, Novartis AG, Johnson & Johnson, and Merck & Co., Inc. are the major players.
What will be the CAGR of the bacterial vaginosis market?
The CAGR of the Bacterial Vaginosis Market is projected to be 8.9% from 2025-2032.