Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Size - Analysis
The Chronic Thromboembolic Pulmonary Hypertension Market is expected to witness positive growth over the next few years. There is no standard treatment available for CTEPH currently. However, the approval and launch of new drugs to treat the disease is anticipated to provide opportunities for growth of the market. In addition, ongoing clinical trials evaluating novel drugs and treatment options for CTEPH will further aid market expansion through 2032.
Market Size in USD Bn
CAGR7.3%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 7.3% |
| Market Concentration | High |
| Major Players | Bayer, SciPharm Sarl, Actelion Pharmaceuticals Ltd, Pfizer, United Therapeutics and Among Others |
please let us know !
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Trends
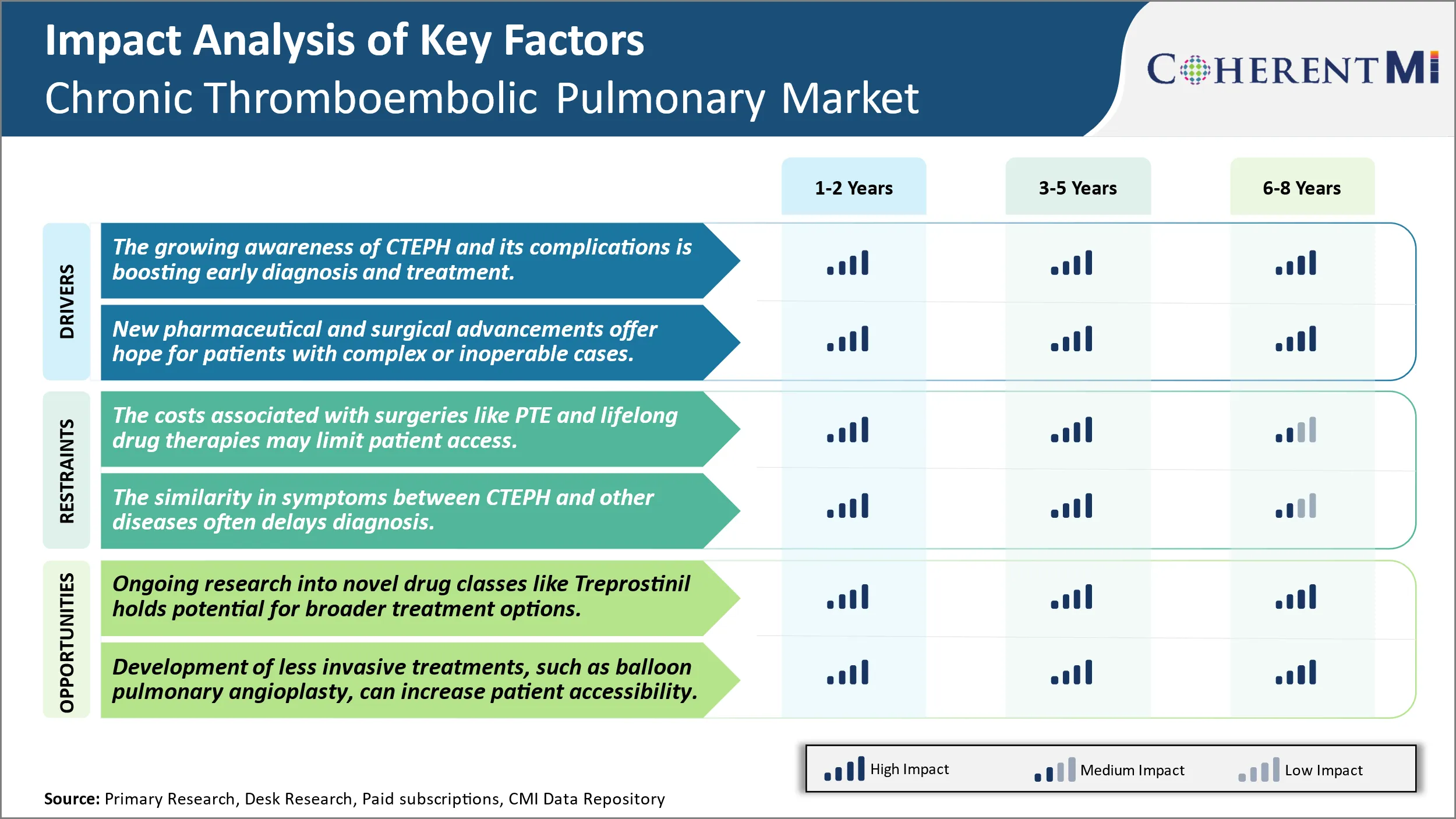
The growing knowledge and understanding about Chronic Thromboembolic Pulmonary Hypertension amongst both patients and physicians have played a crucial role in improving early detection of the disease. In the past, CTEPH often went undiagnosed for longer periods which led to worse health outcomes and higher mortality rates. However, with greater public awareness initiatives by patient advocacy groups and medical organizations in recent years, people are now more informed about the signs and symptoms of CTEPH.
Moreover, many healthcare systems across the developed world have started recognizing CTEPH as a distinct post-thrombotic entity. This official recognition of the disease has helped put it on the radar of more providers and health insurers. It is encouraging further research into CTEPH while also making affordable treatment options accessible to growing number of patients. The growing patient volumes are attracting greater participation of hospitals, doctors and investment from pharmaceutical companies in this therapeutic area. Overall, enhanced awareness of CTEPH as a serious but treatable medical condition if diagnosed early has significantly benefited stakeholders and augmented efforts to study and address this idle disease.
Market Driver - Emergence of New Therapies Fuels the Market Developments.
Likewise, the establishment of percutaneous balloon pulmonary angioplasty for inoperable CTEPH patients has been a game changer. This catheter-based technique uses balloons to literally break up clots inside the lungs through mechanical dilation and restore blood flow. It is proving to be a highly beneficial therapeutic option for a select group of individuals. Ongoing research is further assessing the long-term safety, efficacy and potential of newer alternative treatments such as pulmonary thromboendarterectomy via video-assisted thoracoscopy.
Market Challenge - The Costs Associated with Surgeries Like PTE And Lifelong Drug Therapies May Limit Patient Access.
A bright spot on the horizon for the Chronic Thromboembolic Pulmonary Hypertension Market is the ongoing research into new and improved treatment approaches. One area showing promise is novel drug classes, like the prostacyclin analogue treprostinil. Clinical trials have demonstrated treprostinil's ability to significantly reduce pulmonary arterial pressure and improve functional capacity when administered through subcutaneous injection or infusion. Its favorable side effect profile compared to older therapies like epoprostenol also enhances patient adherence. If future studies continue corroborating treprostinil's safety and efficacy profile, it could become a new standard of care option and help more CTEPH patients achieve meaningful symptomatic control. Beyond treprostinil, pharmaceutical companies and research institutes are exploring modified prostanoids, soluble guanylate cyclase stimulators, and other pipeline candidates. The development of innovative therapies holds potential for offering broader treatment choices that may address unmet needs within select patient subgroups. It could help expand the reach of pharmacologic interventions for CTEPH.
Prescribers preferences of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
Thromboembolic Pulmonary Hypertension (TEPH) can be treated through a stepped approach with multiple lines of therapy. For newly diagnosed cases, or mild TEPH, prescribers typically start with oral medications such as Endothelin receptor antagonists (ERAs). Ambrisentan (Letairis) and macitentan (Opsumit) are commonly prescribed ERAs at this stage.
In severe TEPH cases unresponsive to the above regimens, prescribers generally recommend transitioning to prostacyclin analogs like Riociguat (Adempas), a soluble guanylate cyclase stimulator. It provides an oral option with less invasive administration than prostanoids.
Treatment Option Analysis of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
Thromboembolic pulmonary hypertension (TEPH) has four WHO functional classes (FC) based on symptoms and limitation in physical activity. For FC I-II patients with no or mild limitation, anticoagulation therapy with warfarin is the first-line treatment to prevent new clots.
FC IV patients have severe symptoms at rest requiring parenteral prostanoids. Epoprostenol (Flolan) given as a continuous IV infusion is the gold standard, improving hemodynamics, functional class and survival. Iloprost (Ventavis) can be administered via nebulization 6-9 times daily. Treprostinil (Remodulin) is given subcutaneously via infusion pump.
Key winning strategies adopted by key players of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
The Chronic Thromboembolic Pulmonary Hypertension Market has seen significant growth in recent years due to increased research activities focusing on developing treatments for this life-threatening disease. Leading pharmaceutical companies have adopted collaborative strategies to expand their product pipelines.
Another strategy seen is acquisitions of smaller companies developing novel treatment options. In 2019, Johnson & Johnson acquired PhaseBio for USD2.1 billion to gain rights to its experimental CTEPH therapy bentracimab. This gave J&J early access to a potential blockbuster drug while avoiding long development timelines. Bentracimab is currently in Phase 3 trials and has the potential to replace invasive therapies if approved.
Such strategies of partnerships, acquisitions and supplemental filings have led to a spike in drug approvals in recent years, translating to higher market revenue. Collaborations allow risks and larger patient populations to be addressed, benefiting patients, while supplemental approvals extract additional value from existing brands.
Segmental Analysis of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
-market-by-type-of-treatment.png) Insights, By Type of Treatment Surgical Segment Leads the Bandwagon in the Forecast Period.
Insights, By Type of Treatment Surgical Segment Leads the Bandwagon in the Forecast Period.The surgical treatment segment currently dominates the Chronic Thromboembolic Pulmonary Hypertension Market share with 51.5% in 2025 owing to the inadequacy of pharmacological options for many patients. Pulmonary endarterectomy (PEA) has long been established as the treatment of choice for proximal CTEPH, offering the only potentially curative option. When performed by experienced surgeons at specialized centers, PEA has been shown to significantly improve hemodynamics and functional status in the majority of eligible patients.
The lack of established surgical alternatives for distal CTEPH points to the critical need for pharmacological therapies to treat this patient subset. However, Riociguat is the only approved drug specifically indicated for CTEPH, and long-term data on efficacy is limited. While Riociguat offers an important non-surgical option, its modest hemodynamic effect sizes have left many patients still symptomatic on maximally tolerated therapy. Novel drugs targeting the dysfunctional pathways in CTEPH pathology could widen treatment options beyond Riociguat alone.
-market-by-route-of-administration.png)
Insights, By Route of Administration, Oral Administration Drives Pharmacological Treatment Uptake
The only approved oral therapy Riociguat provides a convenient, outpatient treatment approach administered twice daily with or without food. This dosing flexibility fits well into most patients' daily routines. In contrast, investigational intravenous prostacyclin therapies require complex inpatient initiations and involve frequent hospital visits or home infusions via portable pumps. The inconvenience of IV administration presents challenges to real-world usage that have hindered uptake.
Additional Insights of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) is a rare but severe condition with significant implications for the healthcare system. Despite its rarity, the disease carries a high mortality rate if left untreated, and it often goes undiagnosed due to symptom overlap with other diseases. The market for CTEPH treatment is poised for substantial growth over the coming decade, driven by the introduction of novel therapies, improved diagnostic techniques, and heightened disease awareness. As of now, pulmonary endarterectomy remains the gold standard for treatment, but not all patients are suitable for surgery. For inoperable cases, medical treatments such as ADEMPAS (Riociguat) provide a lifeline, helping to improve hemodynamics and quality of life. Additionally, emerging therapies, such as Treprostinil by SciPharm Sarl, are showing promise in clinical trials. With the increasing number of available treatment options and the anticipated entry of new drugs into the market, the competitive landscape is expected to evolve, offering more opportunities for patients and stakeholders alike.
Competitive overview of Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
The major players operating in the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market include Bayer, SciPharm Sarl, Actelion Pharmaceuticals Ltd, Pfizer, United Therapeutics, AOP Pharmaceuticals AG, GSK Plc, Acceleron Pharma, Jansen Pharmaceuticals and Merck & Co. Inc.
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Leaders
- Bayer
- SciPharm Sarl
- Actelion Pharmaceuticals Ltd
- Pfizer
- United Therapeutics
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market - Competitive Rivalry

Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market
- In May 2024, Bayer launched new clinical trials to extend the use of ADEMPAS to additional patient groups, expanding its market dominance.
- In April 2023, SciPharm Sarl announced positive results in the Phase III trials of Treprostinil, demonstrating improved outcomes for inoperable patients.
Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market Segmentation
- By Type of Treatment
- Surgical Treatment
- Pharmacological Treatment
- Intervention
- Transplantation
- By Route of Administration
- Oral
- Intravenous

Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Frequently Asked Questions :
How Big is the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market?
The Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market is estimated to be valued at USD 1.51 Bn in 2025 and is expected to reach USD 2.47 Bn by 2032.
What will be the CAGR of the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market?
The CAGR of the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market is projected to be 7.11% from 2024 to 2031.
What are the major factors driving the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market growth?
The growing awareness of CTEPH and its complications is boosting early diagnosis and treatment. New pharmaceutical and surgical advancements offer hope for patients with complex or inoperable cases are the major factor driving the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market.
What are the key factors hampering the growth of the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market?
The costs associated with surgeries like PTE and lifelong drug therapies may limit patient access and the similarity in symptoms between CTEPH and other diseases often delays diagnosis. These are the major factors hampering the growth of the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market.
Which is the leading Type of Treatment in the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market?
Surgical Treatment is the leading Type of Treatment segment.
Which are the major players operating in the Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Market?
Bayer, SciPharm Sarl, Actelion Pharmaceuticals Ltd, Pfizer, United Therapeutics, AOP Pharmaceuticals AG, GSK Plc, Acceleron Pharma, Jansen Pharmaceuticals, Merck & Co. Inc are the major players.