Global TIL Therapy Market Size - Analysis
The market is witnessing positive trends that are expected to drive the growth during the forecast period. Since TIL therapy is still new, ongoing clinical trials will help understand its potential better and drive its adoption. Moreover, growing cancer incidence rates will also boost the need for advanced treatment options like TIL therapy worldwide. However, high costs associated with the therapy and lack of expertise may limit the market potential in the coming years.
Market Size in USD Bn
CAGR39.5%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 39.5% |
| Market Concentration | High |
| Major Players | Iovance Biotherapeutics, Instil Bio, Obsidian Therapeutics, Cellectis, Cellular Biomedicine Group and Among Others |
please let us know !
Global TIL Therapy Market Trends
The rising incidence of various types of cancer worldwide has become a serious public health concern. According to estimates, melanoma alone accounts for over 100,000 new cases and over 7,000 deaths each year in the United States. While surgical resection and chemotherapy/radiation therapy have been the frontline interventions for cancer for many years, their effectiveness remains limited, especially in advanced stages. There is a clear clinical need for more targeted and personalized treatment approaches with improved outcomes. This is where TIL cell therapy plays an important role. Several studies have demonstrated the potential of TIL therapy, especially for metastatic melanoma where lymphodepletion followed by TIL infusion has shown response rates of over 50% in clinical trials. Similar results have also been seen in patients with lung and bladder cancer, indicating the broader applicability of this immunotherapy approach. As cancers become more treatment-resistant with current intervention modes, TIL therapy offers promise as a durable treatment alternative that harnesses the body's own immune system to fight tumors in a sustainable manner. With its encouraging efficacy and safety profile established thus far, TIL cell therapy market is expected to flourish further with more research evaluating its use in additional solid tumors like head and neck cancer.
Significant advancements in T-cell immunotherapy, leading to more effective and targeted treatment options:
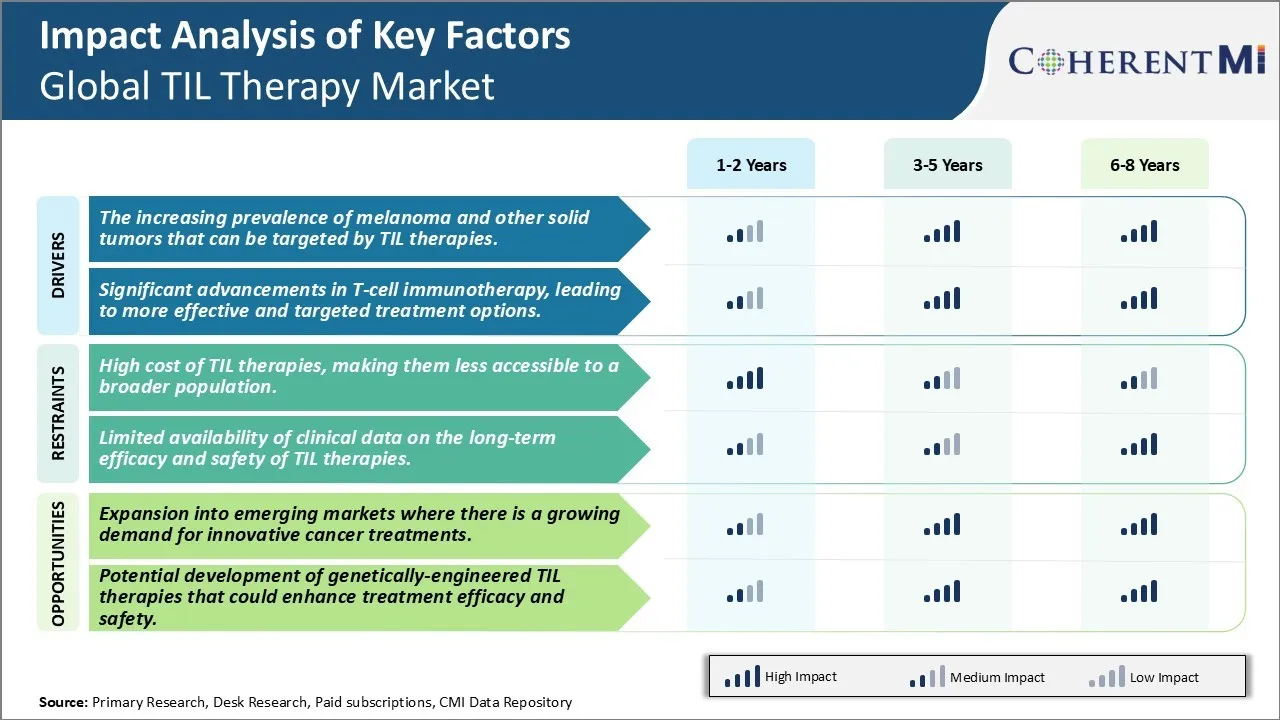
Market Challenge - High cost of TIL therapies, making them less accessible to a broader population
One significant opportunity area for companies in the global TIL therapy market is the expansion of commercialization efforts into emerging markets. While currently approved and reimbursed in only a handful of developed nations, emerging economies in regions such as Asia, Latin America, Middle East, and Africa represent a large and growing market for innovative cancer treatments. As economies in these regions become more advanced, healthcare systems are increasingly able to dedicate more resources toward sophisticated therapies. A growing middle class also means more patients are able to directly pay out-of-pocket or get coverage through private insurance for high-cost treatments. With cancer incidence rates steadily rising globally as populations age, the demand for cutting-edge immunotherapies like TIL cell therapies is expected to ramp up significantly in emerging nations. By launching early commercial and clinical efforts catered to these markets, companies currently leading in TIL therapy development can position themselves to capitalize on future growth opportunities and potentially reach a much wider pool of patients worldwide.
Key winning strategies adopted by key players of Global TIL Therapy Market
Strategic collaborations and partnerships: Companies have formed strategic partnerships with research institutes and hospitals to advance TIL therapy research and clinical trials. For example, in 2017, Immunocore entered into a collaboration with MD Anderson Cancer Center to develop T-cell therapy programs including TIL therapy for multiple cancer types. Such partnerships help companies gain access to new technologies, expertise, and patient populations to accelerate their TIL therapy development programs.
Acquisitions to enhance capabilities: Companies have acquired other firms to enhance their TIL therapy development pipelines and manufacturing capabilities. For instance, in 2018 Roche acquired Foundation Medicine to gain expertise in molecular profiling that can help identify cancer patients most likely to respond to TIL therapy. Such acquisitions allow companies to offer more comprehensive TIL therapy solutions.
Focus on personalized therapy approaches: Companies are devising strategies to deliver personalized TIL therapy products that can be tailored to individual patient's tumor type and genetic profile.
Segmental Analysis of Global TIL Therapy Market
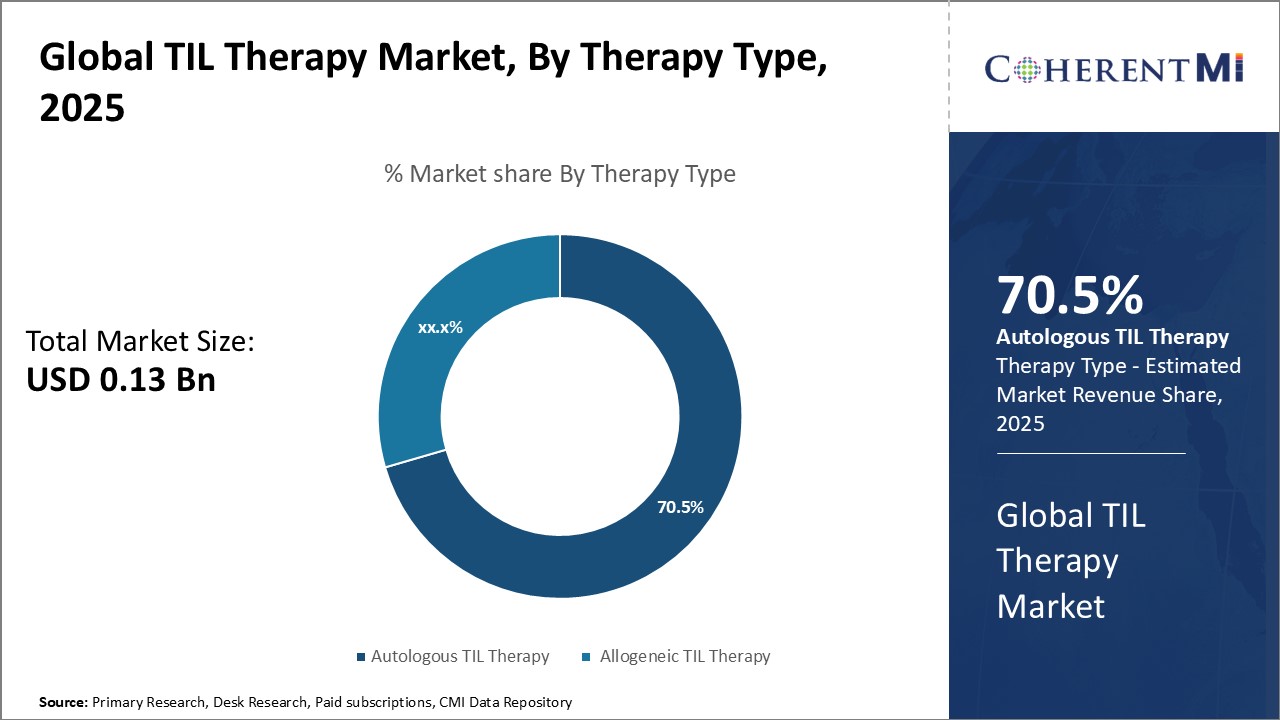 By Therapy Type - Adoption of Autologous TIL Therapy drives its dominant market share
By Therapy Type - Adoption of Autologous TIL Therapy drives its dominant market shareIn terms of By Therapy Type, Autologous TIL Therapy contributes the highest share of the market owning to its greater compatibility with the human immune system when compared to other treatment types. As it uses the patient's own T cells to fight cancer, autologous TIL therapy faces lesser risk of immune rejection. This increased biocompatibility allows for more effective destruction of cancerous cells with relatively mild side effects. Additionally, autologous TIL therapy's personalized approach of extracting T cells directly from each patient makes it a more tailored solution than off-the-shelf allogenic options. Such customization helps maximize the therapy's responsiveness to unique characteristics of each patient's cancer. These advantages over other therapy types have led to greater adoption of and preference for autologous TIL treatment among both medical practitioners and patients.
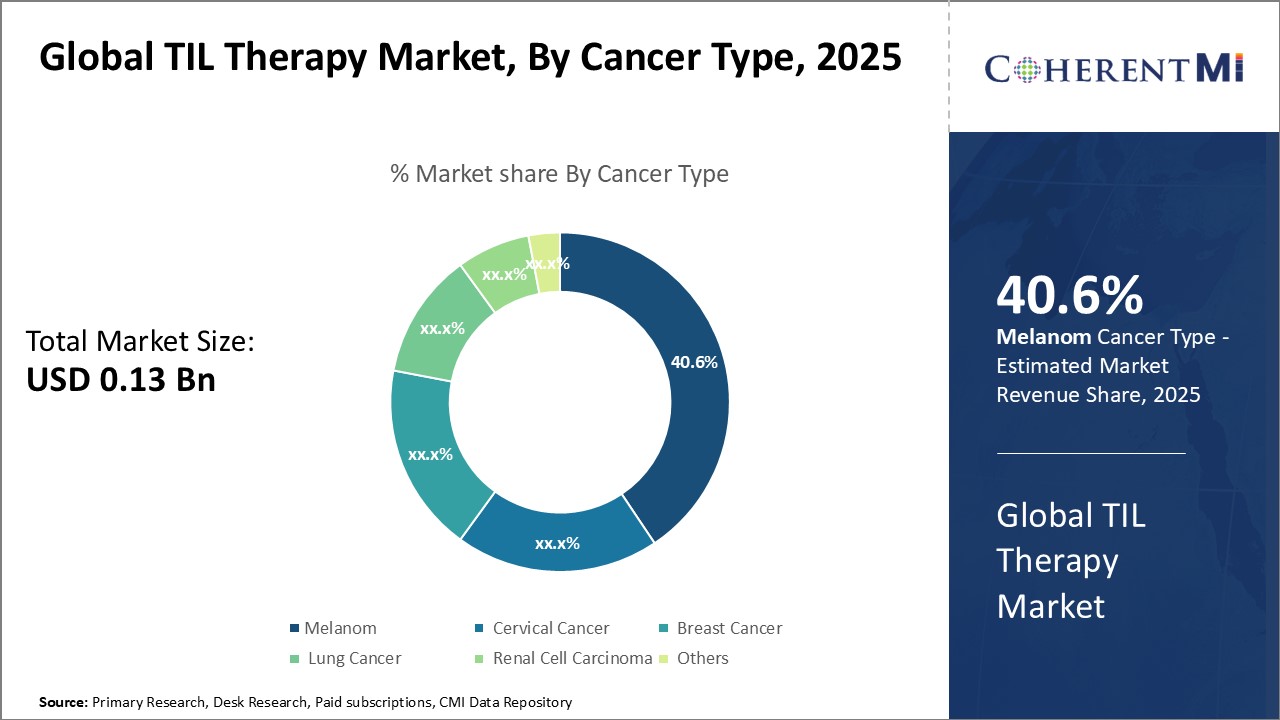
In terms of By Cancer Type, Melanoma contributes the highest share of the market owning to its characteristic presentation of high numbers of tumor-infiltrating lymphocytes (TILs). TILs are immune cells that have migrated from blood vessels into tumors, and their presence indicates the body's natural anti-tumor response. Melanoma tumors exhibit some of the highest levels of TILs among various cancer types. This makes them well-suited for TIL therapy which works by harvesting and multiplying these pre-existing anti-tumor T cells from the tumor microenvironment. The abundance of usable TILs in melanoma increases the therapy's effectiveness for this cancer. In contrast, some other cancer types may provide fewer TILs, challenging treatment efficacy. Therefore, the above-average TIL presence in melanoma drives its prominence as the leading application area for this immunotherapy.
By End User - Concentrated treatment approach boosts hospital segment share
Additional Insights of Global TIL Therapy Market
- The TIL therapy market is poised for significant growth due to advancements in cancer immunotherapy, particularly in the development of tumor-infiltrating lymphocytes (TILs) that target and eliminate tumor cells. With ongoing clinical trials showing promising results, particularly in melanoma and head and neck carcinoma, the market is expected to expand rapidly. The potential for TIL therapies to provide more effective treatment options with fewer side effects than traditional therapies positions them as a critical innovation in oncology. As the market grows, driven by both industry and academic players, the landscape of cancer treatment could be profoundly altered. North America and Europe are expected to dominate the market, with major players like Iovance Biotherapeutics leading the charge. The introduction of genetically-engineered TIL therapies could further enhance the efficacy and safety of these treatments, opening new avenues for cancer care.
Competitive overview of Global TIL Therapy Market
The major players operating in the Global TIL Therapy Market include Lytix Biopharma, Phio Pharmaceuticals, Bristol-Myers Squibb, Incyte Corporation, Kite Pharma, Adaptimmune Therapeutics, Medigene AG, Takeda Pharmaceutical Company, Allogene Therapeutics, Prometheus Laboratories, Century Therapeutics, Arcellx, Immatics, Lyell Immunopharma and CAR-T (Shanghai) Cell Biotechnology.
Global TIL Therapy Market Leaders
- Iovance Biotherapeutics
- Instil Bio
- Obsidian Therapeutics
- Cellectis
- Cellular Biomedicine Group
Global TIL Therapy Market - Competitive Rivalry

Global TIL Therapy Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Global TIL Therapy Market
- On February 2024, Moffitt Cancer Center's innovative cellular immunotherapy, Lifileucel, has received FDA approval and is now available for patients with advanced melanoma. Lifileucel is the first tumor-infiltrating lymphocyte (TIL) therapy approved for solid tumors. The therapy involves surgically removing melanoma tumors, extracting and multiplying the immune cells, and then infusing them back into the patient after chemotherapy.
- In August 2023: Phio Pharmaceuticals, in collaboration with AgonOx and Providence Cancer Institute, announced the dosing of the first patient with melanoma and other advanced solid tumors using AGX148 'double positive' CD8 tumor-infiltrating lymphocytes alone and in combination with Phio's PD-1 silencing PH-762.
- July 2023: Iovance Biotherapeutics received positive regulatory and clinical updates for IOV-LUN-202, aiming for accelerated approval of TIL therapy in post-anti-PD-1 advanced non-small cell lung cancer (NSCLC).
- In May 2023: Iovance Biotherapeutics announced the FDA acceptance of its biologics license application (BLA) for lifileucel, which also received priority review status
Global TIL Therapy Market Segmentation
- By Therapy Type
- Autologous TIL Therapy
- Allogeneic TIL Therapy
- By Cancer Type
- Melanoma
- Cervical Cancer
- Breast Cancer
- Lung Cancer
- Renal Cell Carcinoma
- Others (Gastrointestinal Cancers, Head and Neck Cancers, etc.)
- By End-User
- Hospitals
- Cancer Research Institutes
- Specialty Oncology Clinics
- Others

Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Frequently Asked Questions :
How big is the Global TIL Therapy Market?
The Global TIL Therapy Market is estimated to be valued at USD 0.13 in 2025 and is expected to reach USD 1.34 Billion by 2032.
What are the major factors driving the Global TIL Therapy Market growth?
The the increasing prevalence of melanoma and other solid tumors that can be targeted by til therapies. and significant advancements in t-cell immunotherapy, leading to more effective and targeted treatment options. are the major factor driving the Global TIL Therapy Market.
Which is the leading Therapy Type in the Global TIL Therapy Market?
The leading Therapy Type segment is Autologous TIL Therapy.
Which are the major players operating in the Global TIL Therapy Market?
Lytix Biopharma, Phio Pharmaceuticals, Bristol-Myers Squibb, Incyte Corporation, Kite Pharma, Adaptimmune Therapeutics, Medigene AG, Takeda Pharmaceutical Company, Allogene Therapeutics, Prometheus Laboratories, Century Therapeutics, Arcellx, Immatics, Lyell Immunopharma, CAR-T (Shanghai) Cell Biotechnology are the major players.
What will be the CAGR of the Global TIL Therapy Market?
The CAGR of the Global TIL Therapy Market is projected to be 39.5% from 2025-2032.