Hand Foot Syndrome Market Size - Analysis
Market Size in USD Mn
CAGR6.7%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 6.7% |
| Market Concentration | Medium |
| Major Players | Pfizer Inc., Roche Holding AG, Novartis International AG, Merck & Co., Inc., Johnson & Johnson and Among Others |
please let us know !
Hand Foot Syndrome Market Trends
As cancer being one of the leading causes of mortality worldwide, the need for effective treatment methods has grown exponentially. Chemotherapy has established itself as one of the foremost lines of treatment against cancer, successfully able to extend lives and achieve remission. However, chemotherapy drugs are not without side effects and one such common side effect is hand-foot syndrome.
With global cancer figures projected to continue ascension in coming years owing to aging population and lifestyle changes, chemotherapy treatments shall remain indispensable. This will maintain pressure on drug manufacturers as well as health systems to effectively manage the side effects. As long as chemotherapy usage remains predominant, related adverse effects like hand foot syndrome will persist as a challenge.
Market Driver - Growing Awareness and Demand for Improved Treatment Options
Hand-foot syndrome, as one such side effect of selected chemotherapy drugs, has seen increased visibility through various cancer support groups and advocacy platforms. Patients are more informed now about the possibility of developing skin irritations on hands and feet during or after chemotherapy cycles.
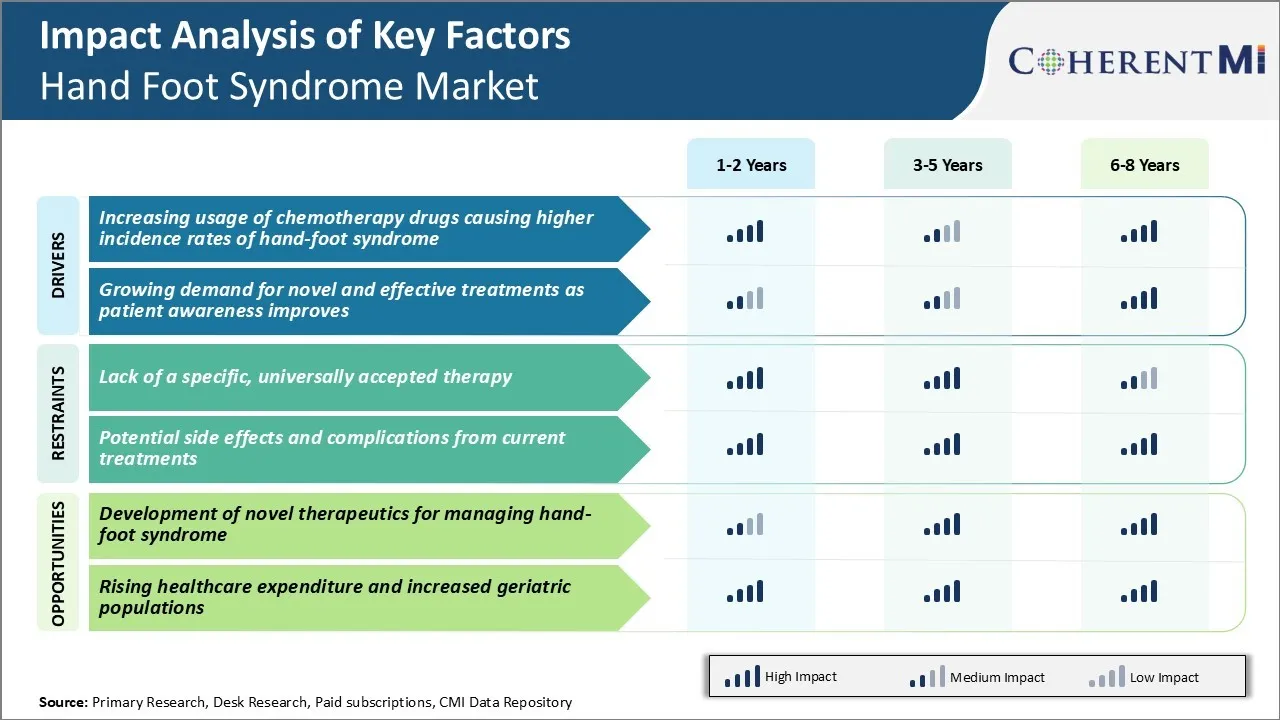
Market Challenge - Lack of a Specific, Universally Accepted Therapy
No current options have demonstrated clear superiority over others or provided long lasting relief for patients. This uncertainty surrounding the optimal treatment approach leads to inconsistent care for patients and difficulty designing clinical trials.
The current lack of effective therapeutic options for hand-foot syndrome presents a major market opportunity for developing novel drug candidates. Successful introduction of new medications that can reliably prevent onset or rapidly resolve symptoms would capture significant market share by addressing this significant unmet medical need.
Additionally, novel biological insights enabling patient stratification hold promise to elucidate the pathophysiology of vulnerable subsets and their differing response to investigational therapies. Such precision medicine approaches may accelerate clinical development by enriching enrollment for responsive patient populations most likely to benefit.
Prescribers preferences of Hand Foot Syndrome Market
Hand foot syndrome (HFS), also known as palmar-plantar erythrodysesthesia, is a common side effect of certain chemotherapy drugs. Prescribers typically follow a stepwise approach based on the severity of symptoms.
For moderate HFS, prescription-strength topical steroids are used. Popular options include mometasone (Elocon) and clobetasol (Temovate). Cooling gels containing menthol or camphor (Aspercreme) provide symptomatic relief. Antihistamines like diphenhydramine (Benadryl) may alleviate itching.
The stage of the chemotherapy regimen and the patient's overall cancer prognosis strongly influence prescribers' line of treatment for HFS.
Treatment Option Analysis of Hand Foot Syndrome Market
HFS has four stages ranging from mild to severe based on severity of symptoms. Stage 1 or mild HFS involves only pain with no ulceration. Stage 2 involves pain with erythema and swelling without ulceration. Stage 3 has pain with blistering and ulceration less than 2cm, while Stage 4 presents pain with severe ulceration above 2cm.
Stage 3 warrants more potent interventions. Topical calcineurin inhibitors like pimecrolimus or topical corticosteroid combination therapies provide relief in over 60% cases. Alternatively, targeted therapies like palmar-plantar erythrodysesthesia cream containing dyclonine, hydrogenated rosins, and pramoxine hydrochloride help prevent progression to Stage 4.
Key winning strategies adopted by key players of Hand Foot Syndrome Market
Companies have focused on developing novel drug formulations to effectively manage the symptoms of hand foot syndrome. For example, in 2018, Eisai gained FDA approval for Lenvima oral capsules for the treatment of refractory differentiated thyroid cancer. Lenvima demonstrated statistically significant improvement in progression-free survival compared to placebo with a lowered risk of developing hand foot syndrome. This novel formulation helped Eisai gain a competitive advantage in the hand foot syndrome market.
Companies also focus on expanding into new therapy areas and obtaining additional approvals for existing drugs. In 2020, Jazz Pharmaceuticals received FDA approval for Rybelsus for chronic weight management. Rybelsus is the first and only glucagon-like peptide-1 (GLP-1) receptor agonist approved in tablet form. The oral formulation significantly lowers the risk of hand foot syndrome compared to injectable drugs. This additional approval expanded Rybelsus' total addressable patient population.
Segmental Analysis of Hand Foot Syndrome Market
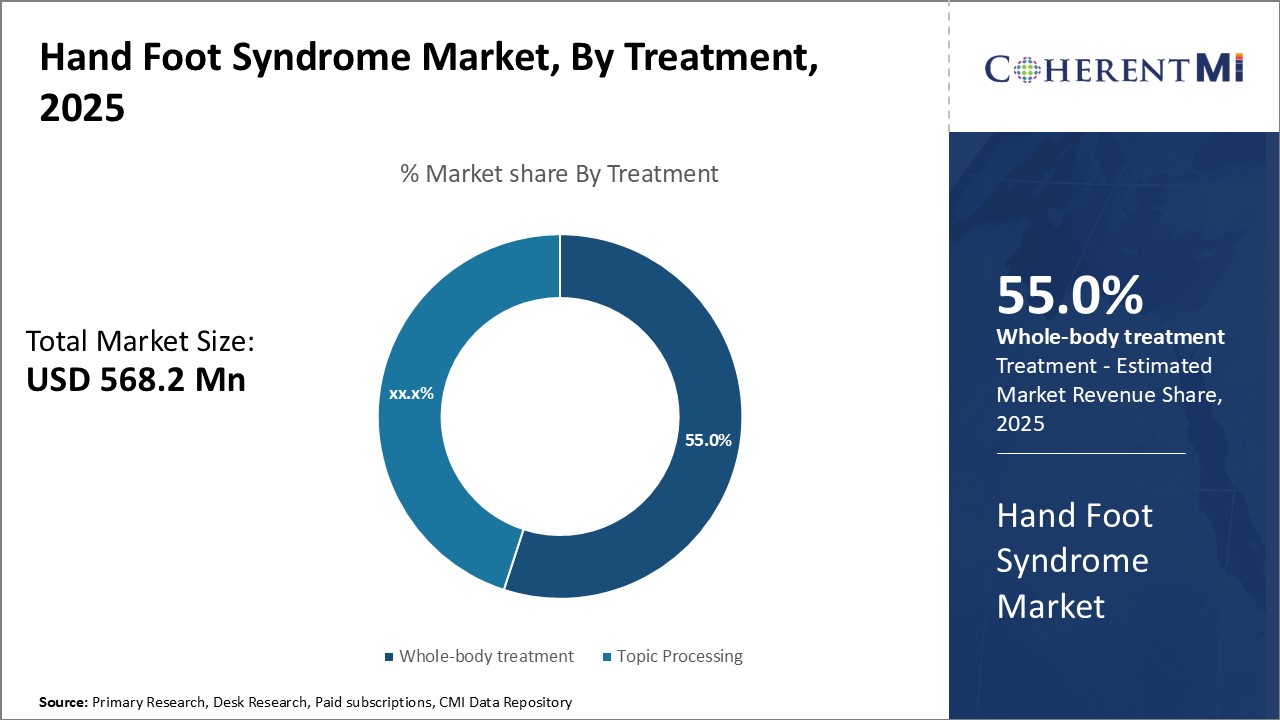 Insights, By Treatment: Systemic Treatments Drive Market Share in Treatment Segment
Insights, By Treatment: Systemic Treatments Drive Market Share in Treatment SegmentSystemic treatments are estimated to account for 55% share of the hand foot syndrome market in 2025, due to their effectiveness in managing underlying causes of the condition. Many cases of Hand Foot Syndrome are related to chemotherapy used in oncology. Systemic treatments work by addressing the patient's overall health and inhibiting factors that may exacerbate symptoms.
However, topical treatments still play an important supportive role by providing symptom relief and preventing secondary infections on the skin. Creams and ointments containing agents like urea, aqueous cream, and treatex help moisturize dry areas and form protective barriers. The focus on addressing causal factors pharmacologically through absorption into the bloodstream gives systemic treatments an edge over surface-level options. It positions them as the primary line of defense in serious cases tied to systemic diseases. Their superiority in managing disease progression rather than just symptoms contribute significantly to systemic therapies' pole position in the treatment segment.
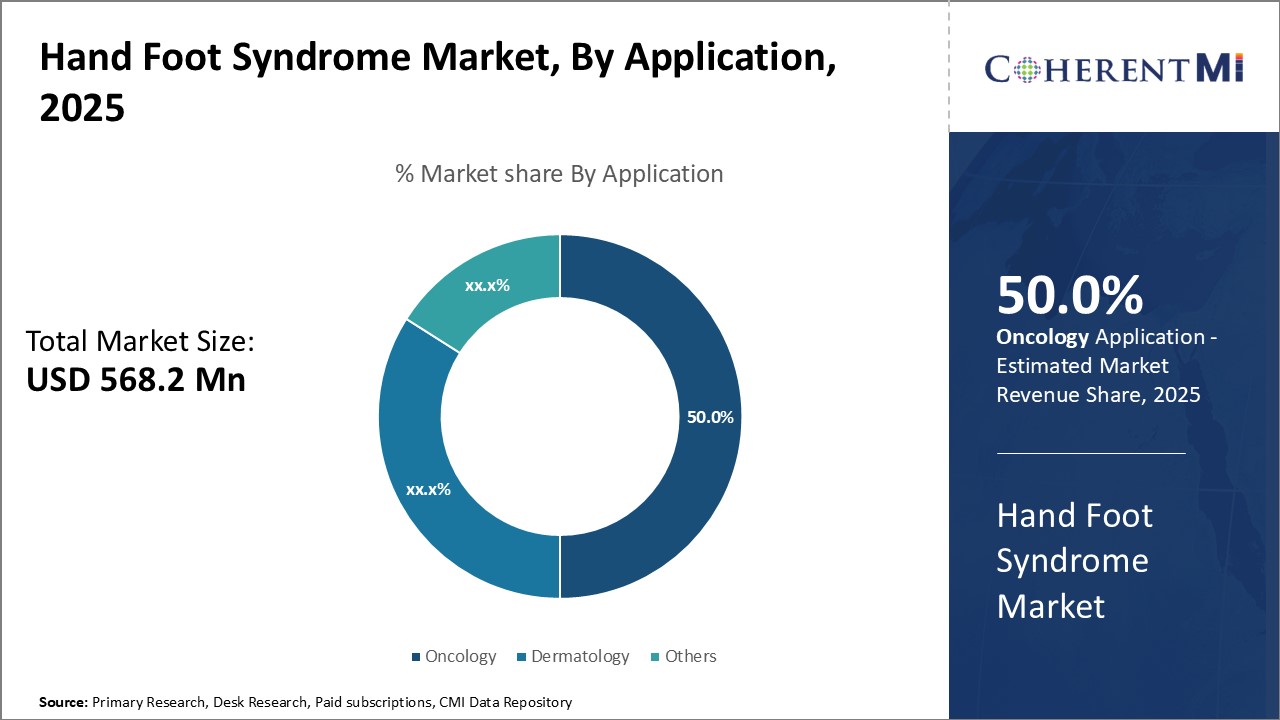
Oncology is estimated to account for 50% share of the hand foot syndrome market in 2025, in terms of application, mainly because chemotherapy is a leading cause of the condition. A sizeable portion of Hand Foot Syndrome cases stem from cytotoxic agents administered as part of cancer treatment regimens. Chemotherapeutics interfere with DNA synthesis, which can damage skin cells and produce symptoms like redness, swelling, and desquamation.
While dermatology and supportive care also contribute applications, the sheer scale of chemotherapy use in fighting cancer means oncology dwarfs other areas. The direct links between chemo agents and hand foot syndrome etiology cement oncology's dominant position. Ensuring cancer patients can complete treatment courses without interruptions from skin issues is a compelling driver of demand within this application segment.
Additional Insights of Hand Foot Syndrome Market
- Prevalence Rates: Approximately 30% of patients undergoing chemotherapy experience Hand-Foot Syndrome, underscoring the need for effective management solutions.
- Regional Growth: Asia-Pacific is expected to witness the highest growth rate in the HFS market due to rising cancer incidence and improving healthcare infrastructure.
- Treatment Adoption: Topical treatments currently hold the largest market share, accounting for 40% of the total market.
- The incidence of hand-foot syndrome is highest among patients receiving VEGFR inhibitors, particularly in the U.S. market. This accounts for about 65% of all cases.
Competitive overview of Hand Foot Syndrome Market
The major players operating in the hand foot syndrome market include Pfizer Inc., Roche Holding AG, Novartis International AG, Merck & Co., Inc., Johnson & Johnson, Quanta Medical, Pfizer, Hoffmann-La Roche, Merck Sharp & Dohme LLC, Ortho Biotech Inc., and Nordic Pharma SAS.
Hand Foot Syndrome Market Leaders
- Pfizer Inc.
- Roche Holding AG
- Novartis International AG
- Merck & Co., Inc.
- Johnson & Johnson
Hand Foot Syndrome Market - Competitive Rivalry

Hand Foot Syndrome Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Hand Foot Syndrome Market
- In August 2024, Bristol-Myers Squibb launched a new topical treatment to mitigate hand-foot syndrome side effects for patients undergoing VEGFR inhibitors treatment. There is ongoing research and some reports about the use of other topical treatments, such as diclofenac gel, in mitigating hand-foot syndrome caused by chemotherapy agents like capecitabine. These advancements are part of broader efforts by the company to improve the management of this side effect.
- In July 2024, Roche Holding AG entered a strategic partnership with a biotech firm to develop advanced systemic treatments for HFS, focusing on minimizing side effects associated with chemotherapy. Recent significant partnerships involving Roche in 2024 include collaborations with companies like Ascidian Therapeutics to develop gene therapies for neurological diseases and MOMA Therapeutics for oncology treatments focused on dynamic protein targets.
- In March 2024, Novartis announced a partnership with local manufacturers in Europe to expand access to cost-effective hand-foot syndrome treatments. Novartis has also been involved in various collaborations and expansions globally, particularly in pharmaceutical manufacturing and research in other areas, such as radioligand therapy and oncology treatments.
Hand Foot Syndrome Market Segmentation
- By Treatment
- Systemic Treatments
- Oral Medications
- Intravenous Therapies
- Topical Treatments
- Creams
- Ointments
- Systemic Treatments
- By Application
- Oncology
- Chemotherapy-induced HFS
- Other Oncology Treatments
- Dermatology
- Chronic Skin Conditions
- Other Dermatological Uses
- Others
- Alternative Therapies
- Supportive Care
- Oncology

Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Frequently Asked Questions :
How big is the hand foot syndrome market?
The hand foot syndrome market is estimated to be valued at USD 568.2 Mn in 2025 and is expected to reach USD 894.7 Mn by 2032.
What are the key factors hampering the growth of the hand foot syndrome market?
The lack of a specific, universally accepted therapy and potential side effects and complications from current treatments are the major factors hampering the growth of the hand foot syndrome market.
What are the major factors driving the hand foot syndrome market growth?
The increasing usage of chemotherapy drugs causing higher incidence rates of hand-foot syndrome and growing demand for novel and effective treatments as patient awareness improves, are the major factors driving the hand foot syndrome market.
Which is the leading treatment in the hand foot syndrome market?
The leading treatment segment is systemic treatments.
Which are the major players operating in the hand foot syndrome market?
Pfizer Inc., Roche Holding AG, Novartis International AG, Merck & Co., Inc., Johnson & Johnson, Quanta Medical, Pfizer, Hoffmann-La Roche, Merck Sharp & Dohme LLC, Ortho Biotech Inc, and Nordic Pharma SAS are the major players.
What will be the CAGR of the hand foot syndrome market?
The CAGR of the hand foot syndrome market is projected to be 6.7% from 2025-2032.