Pharmaceutical Caps And Closures Market Size - Analysis
The pharmaceutical caps and closures market is estimated to be valued at USD 6.84 Bn in 2025 and is expected to reach USD 10.91 Bn by 2032, growing at a compound annual growth rate (CAGR) of 6.9% from 2025 to 2032. The increasing production of pharmaceutical drugs and investments in technological advancements of caps and closures by leading manufacturers are expected to drive the growth of the market during the forecast period.
The market is expected to witness positive growth owing to rising demand for convenient and efficient dosage forms. Further, the introduction of anti-counterfeiting and child-resistant packaging technologies will support market expansion. However, availability of alternative packaging formats and increasing preference for generic drugs may impede the market progression to some extent over the forecast years.
Market Size in USD Bn
CAGR6.9%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 6.9% |
| Market Concentration | High |
| Major Players | Aptar Pharma (AptarGroup), West Pharmaceutical Services, Datwyler Sealing Solutions (Datwyler Group), Lonstroff (Sumitomo Rubber Industries), Ompi (Stevanato Group) and Among Others |
please let us know !
Pharmaceutical Caps And Closures Market Trends
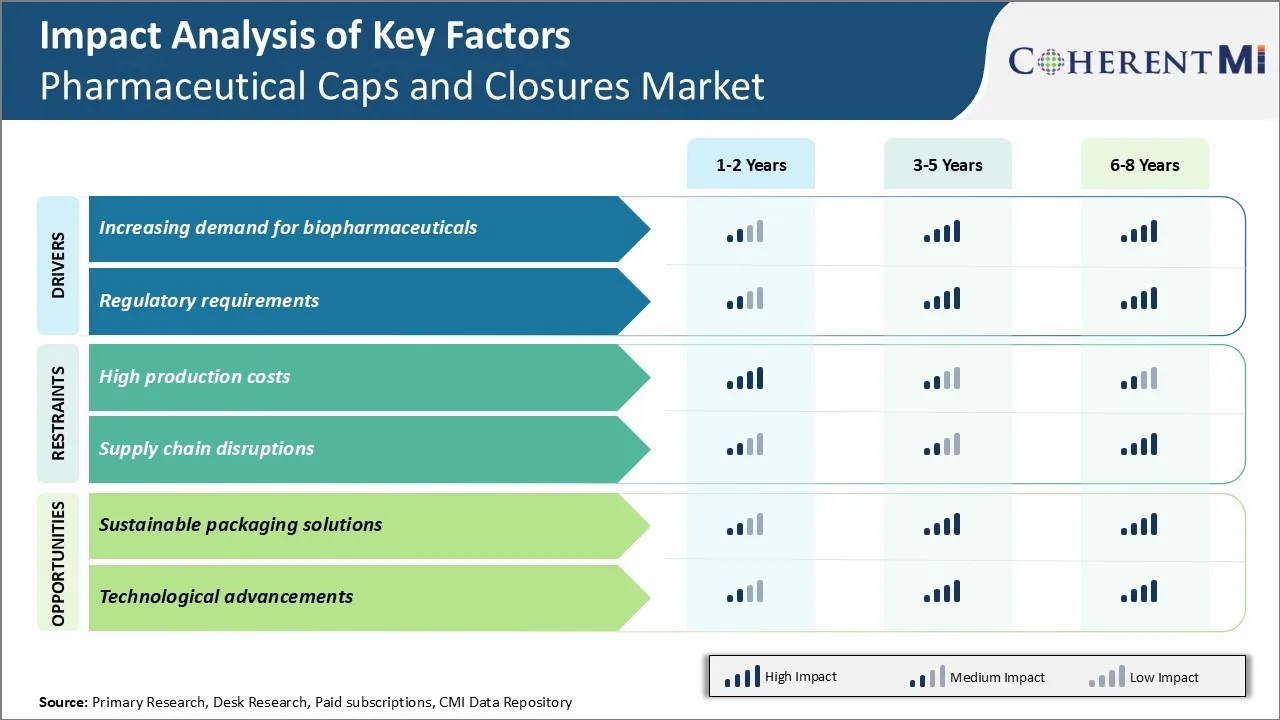
Market Driver - Increasing demand for biopharmaceuticals
As biopharmaceutical products like vaccines and therapies derived from living cells are on the rise, it is boosting the demand for pharmaceutical caps and closures. These biologic drugs require sophisticated packaging solutions to maintain their integrity and efficacy throughout the supply chain. Compared to traditional small molecule drugs, biopharmaceuticals are complex and sensitive to temperature variations. They need secure, tamper-evident and hermetic packaging to prevent contamination and ensure product viability during transportation and storage.
Caps and closures play a vital role in providing effective protection and easy identification of biologic drugs. Advanced container closure systems with moisture barriers, oxygen scavengers and desiccants are becoming increasingly popular for biopharmaceutical packaging. Leak-proof and tamper-proof bottle caps, vial seals and stoppers made of specialized materials like rubber ensure the containment, integrity and stability of temperature-sensitive biologics. Some biopharmaceutical products also require delivery devices like pre-filled syringes that utilize customized closures or stoppers.
The rise of personalized medicine has also fueled the demand for targeted biologic drugs like monoclonal antibodies, recombinant proteins and stem cell therapies. Manufacturers packaging such niche biopharmaceuticals rely on innovative closure designs that enhance handling and allow for resealing. This enables break-up of doses and maintenance of sterility. Further, as biologic drugs approved for self-administration increase, user-friendly child-resistant and senior-friendly packaging with easy-open, easy-close closures are gaining prominence.
With the biopharmaceutical industry expected to grow at a higher pace compared to traditional small molecule drugs, pharmaceutical caps and closures manufacturers are developing advanced solutions suitable for the whole gamut of biologic products. Features like customized tamper-evidence, leakproof seals and ease of use will be vital for successful packaging of the rising category of large molecule drugs in the future.
Market Driver - Regulatory requirements
Stringent regulations surrounding pharmaceutical packaging have always driven innovations and developments in caps and closures. However, the regulatory landscape is now more complex with new guidelines addressing globalization and safety aspects. Manufacturers must comply with the cGMP standards of major markets like the US, Europe and Asia to ensure product quality and prevent counterfeiting across international supply chains.
Additionally, regulations prescribe strict material specifications and testing standards for pharmaceutical packaging. Qualification of components like liners and seals involves sophisticated analytical methods and challenges closure makers to use only FDA-compliant raw materials. Traceability has also become crucial with serialization and aggregation requirements being implemented worldwide. Caps and closures must uniquely identify drugs at the lowest distribution level using technologies like numeric codes and 2D datamatrix barcodes.
Mounting focus on medication errors and misuse has led to new mandates for user-friendly and senior-friendly packaging designs. Child-resistant packaging is now regulated for more drug categories and updated international standards for ease-of-use and ease-of-opening are prompting innovative solutions. Further, regulations now emphasize on "green" and sustainable packaging materials with reduced environmental footprint throughout the product lifecycle. Biodegradable polymers and recyclable materials are gaining ground.
Upcoming regulations like Falsified Medicines Directive in Europe will also involve advanced authentication technologies for pharmaceutical packaging. As regulations evolve dynamically across the globe to align with new healthcare challenges, the pharmaceutical caps and closures industry must consistently upgrade products, manufacturing technologies and quality systems to remain compliant. These drives continued innovation.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - High production costs
The pharmaceutical caps and closures market faces significant challenges in regards to high production costs. Manufacturing pharmaceutical caps and closures requires stringent quality control measures and compliance with good manufacturing practices in order to ensure product and public safety. This involves extensive testing, certifications and specialized machinery. As a result, production takes longer and involves higher costs compared to regular plastic packaging. Additionally, materials used such as aluminum and other metals add to expenses. Regulatory expectations on sterilization such as gamma irradiation further drives up costs. Meeting individual pharmaceutical and biologics companies' customized requirements for packages in terms of colors, sizes etc. also affect economies of scale. These factors make it difficult for manufacturers to keep prices low and compete with alternatives such as blister packs. High costs potentially pose a roadblock for the market's growth especially in price-sensitive and cost-crunched developing healthcare markets.
Market Opportunity - Sustainable packaging solutions
The pharmaceutical caps and closures market sees significant opportunity in developing sustainable packaging solutions. Increased global emphasis on environmental protection has led pharmaceutical manufacturers to focus on green technologies and recyclability. Closure manufacturers catering customized sustainable product offerings can gain competitive advantage. For example, offerings utilizing materials that reduce source dependency on petroleum or are made from recycled content appeal to both pharmaceutical producers and conscious consumers. Solutions incorporating eco-friendly features such as minimal plastic usage, recyclable aluminum caps etc. also meet industry demand. Transitioning to renewable energy sources in factories further boosts sustainability credentials. Caps and closures with efficient protective properties but lower carbon footprint will appeal to healthcare providers and patients concerned about reducing environmental impact of medical waste. Focus on innovative sustainable packaging presents lucrative opportunities for market players to experience growing demand and cement their leadership.
Key winning strategies adopted by key players of Pharmaceutical Caps And Closures Market
Players like West Pharmaceutical Services, Capsugel (Lonza), AptarGroup, etc. have seen great success through continuous investment and focus on innovation. For example, West launched smart dose technology for inhaled respiratory drugs in 2018 and easy open vial technology in 2017. These new product offerings helped them gain market share.
Players provide highly customized solutions according to specific client needs. For example, Capsugel launched Vcaps customized capsules filled with precise dosages of pharmaceutical ingredients in 2015. These are tailored for clients' formulations. Their ability to customize offerings has helped win big contracts.
Strategic M&A allows players to expand capabilities and footprints. In 2016, West acquired Daikyo Seiko which helped them expand in polymer material and closures. This acquisition strengthened their position in the Japanese pharma market. Similarly, Capsugel was acquired by Lonza in 2017 to create an integrated solutions powerhouse.
Players have strategically expanded to high-growth emerging markets like Asia, Latin America, Middle East, etc. through joint-ventures, new plants and acquisitions. For example, in 2018, Capsugel commenced operations in a new facility in China. Their focus on emerging high-growth markets has boosted sales volumes.
Players ensure compliance with evolving global pharmaceutical regulations through continuous upgrades and quality certifications. Their commitment to quality and compliance helps clients feel assured and continues repeat business.
Segmental Analysis of Pharmaceutical Caps And Closures Market
 To learn more about this report, Download Free Sample Copy
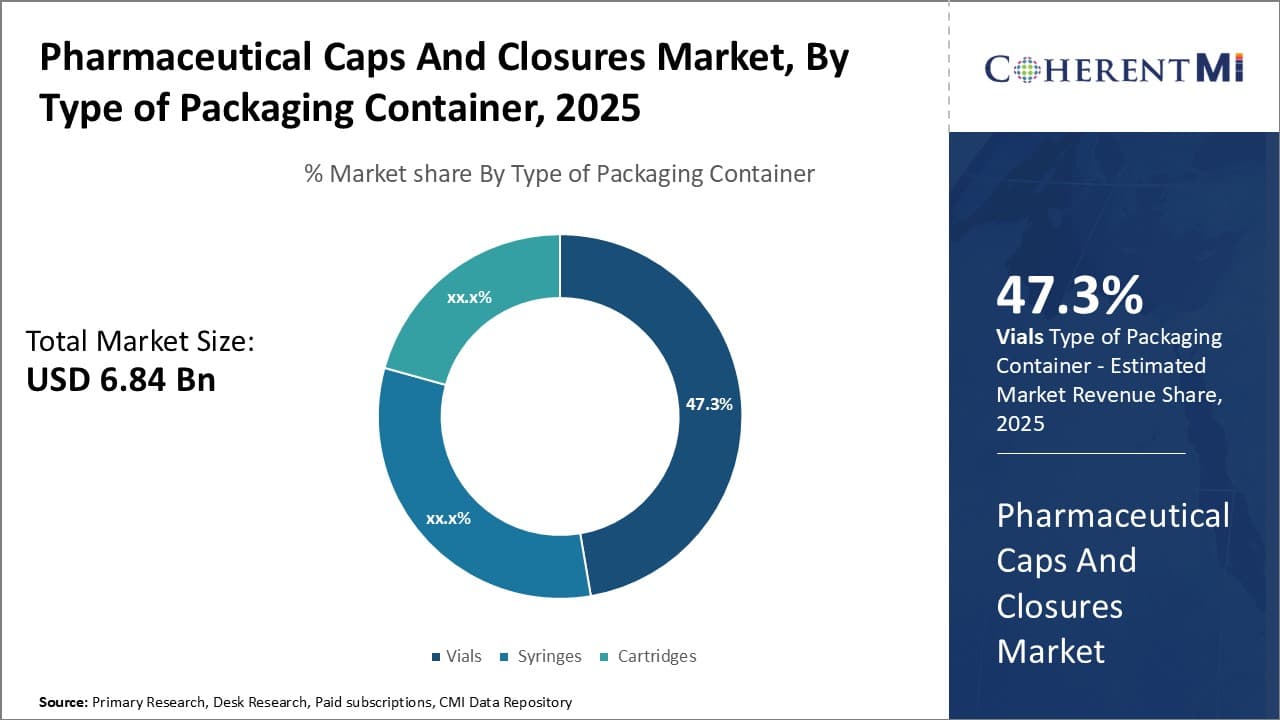
Insights, By Type of Packaging Container: High usability and cost-effectiveness of vials
To learn more about this report, Download Free Sample Copy
Insights, By Type of Packaging Container: High usability and cost-effectiveness of vials
In terms of type of packaging container, vials sub-segment contributes the highest share of 47.3% in the market owing to its high usability and cost-effectiveness. The vials packaging container segment holds the largest share in the pharmaceutical caps and closures market due to its versatility and cost advantages. Vials are slim glass or plastic bottles that are widely used for packaging liquids, creams and similar products. They are highly suited for medications like vaccines, intravenous drugs, eye/ear drops and other small volume liquid formulations.
Vials provide reliable barrier protection against moisture, gases and contaminants. Their seamless structure prevents any ingression of external particles or leakage of contents. This makes vials ideal for packaging sensitive drugs. The cylindrical shape of vials allows for maximum storage capacity within minimum space. They can be easily transported and stored without risk of breakage.
Moreover, vials manufacturing requires relatively less material as compared to other containers like bottles or cartridges. This translates to lower production costs. Vials offer cost-efficient solutions especially for smaller drug volumes. Their production can also be easily automated for mass manufacturing. These cost advantages along with vials' adaptability to liquid drug types drive their higher utilization over alternative packaging types.
The simplicity of vial design also facilitates easy handling and dispensing. No additional devices like syringes or droppers are required for administration of vial contents. This eases administration especially for self-medication products. Overall, vials stand out as a highly functional and affordable packaging option, therefore accounting for the largest demand.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
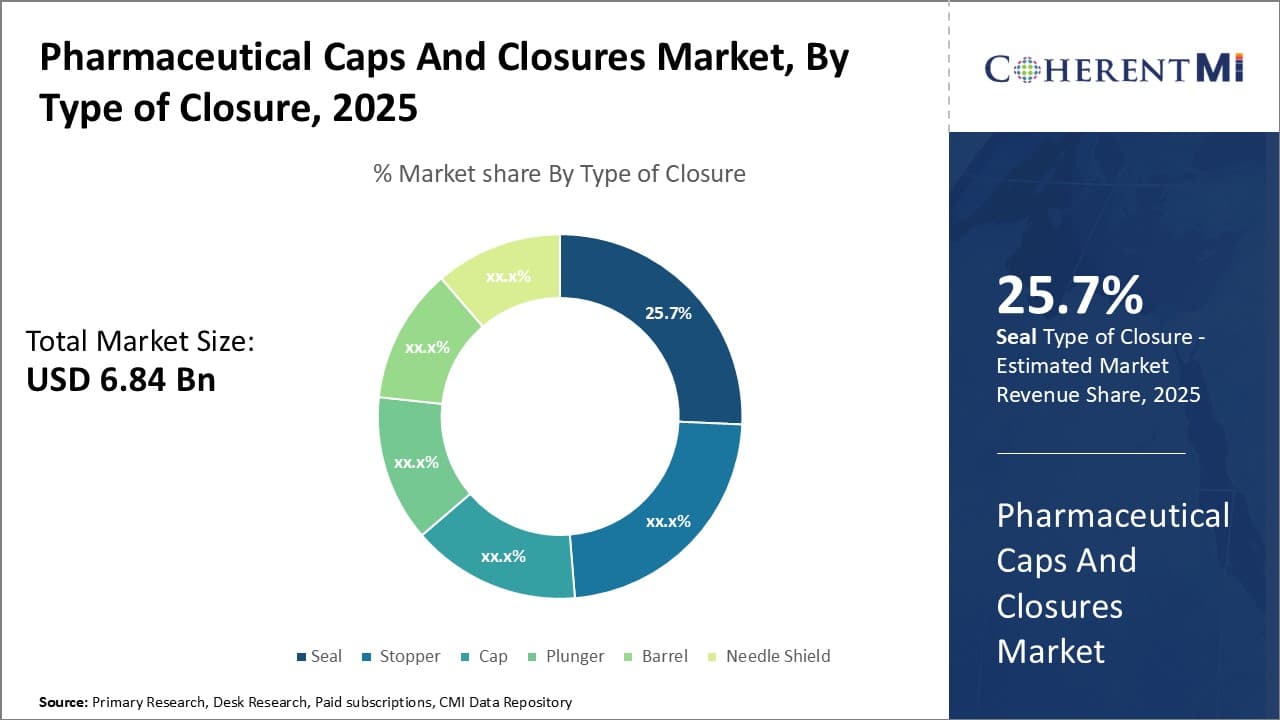
Insights, By Type of Closure: Tamper-proof and leakage prevention properties of seals
Seal sub-segment contributes the highest share of 25.7% in the closures segment owing to its tamper-proof and leakage prevention properties. The seal closure type enjoys a significant market share dominance in the pharmaceutical caps and closures segment attributed to its tamper-proof and leakage prevention abilities. Seals are adhesive or heat sealing foils used to securely close the openings of pharmaceutical containers.
Seals create reliable air-tight seals that protect the product from external contamination and leakage. They prevent entry of moisture, oxygen or microbes into the sealed container. This helps maintain the safety, sterility and long-term stability of packaged medications. Tamper-proof seals also assure customers of product authenticity and integrity.
Moreover, seals can be easily inspected for any signs of previous opening. This allows traceability and helps avoid medication mix-ups. Their straightforward structure and application process contributes to higher production rates and lower costs compared to complex closure types. Film coating technology further enhances the barrier functions of seals.
Seals are highly versatile in usage across diverse drug formulations packaged in various containers including vials, ampoules, syringes and bottles. The self-adhesive nature of seals enables direct application without requiring any other closure apparatus. Their conformability to different container shapes and sizes broadens the addressable market. All these advantages drive higher market preference for seals over other closure types in the pharmaceutical industry.
Additional Insights of Pharmaceutical Caps And Closures Market
- The pharmaceutical caps and closures market is undergoing significant growth driven by the demand for safer and more reliable packaging solutions in the biopharmaceutical industry. The market is also influenced by the shift towards personalized medicine, which requires more specialized packaging solutions. Emerging trends such as automation in manufacturing and sustainable packaging solutions are also shaping the future of the market. Major players are focusing on expanding their product portfolios and geographical reach to maintain their competitive edge.
Competitive overview of Pharmaceutical Caps And Closures Market
The major players operating in the pharmaceutical caps and closures market include Aptar Pharma (AptarGroup), West Pharmaceutical Services, Datwyler Sealing Solutions (Datwyler Group), Lonstroff (Sumitomo Rubber Industries), Ompi (Stevanato Group), Daikyo Seiko, Hebei First Rubber Medical Technology and Jiangsu Hualan New Pharmaceutical Material.
Pharmaceutical Caps And Closures Market Leaders
- Aptar Pharma (AptarGroup)
- West Pharmaceutical Services
- Datwyler Sealing Solutions (Datwyler Group)
- Lonstroff (Sumitomo Rubber Industries)
- Ompi (Stevanato Group)
Recent Developments in Pharmaceutical Caps And Closures Market
- In June 2023, Aptar Pharma launched a new elastomeric closure designed for high potency drugs, enhancing drug safety and reducing contamination risks.
- In May 2023, West Pharmaceutical expanded its production capacity in North America to meet the rising demand for pre-sterilized closures.
- In April 2023, Datwyler entered a strategic partnership to develop sustainable packaging solutions for biologics.
Pharmaceutical Caps And Closures Market Segmentation
- By Type of Packaging Container
- Vials
- Syringes
- Cartridges
- By Type of Closure
- Seal
- Stopper
- Cap
- Plunger
- Barrel
- Needle Shield
Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
