South Korea Car-T Cell Therapy Market Size - Analysis
The South Korea Car-T Cell Therapy Market is estimated to be valued at USD 9.2 Mn in 2025 and is expected to reach USD 26.5 Mn by 2032, growing at a compound annual growth rate (CAGR) of 16.3% from 2025 to 2032.
Market Size in USD Mn
CAGR16.3%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 16.3% |
| Market Concentration | Medium |
| Major Players | Novartis AG, Pfizer, Inc., Bristol Myers Squibb, CARsgen Therapeutics Co., Ltd., Fate Therapeutics, Inc. and Among Others |
please let us know !
South Korea Car-T Cell Therapy Market Trends
CAR-T cell therapy is showing very promising results in treating various difficult-to-treat cancers in South Korea. The high efficiency of CAR-T cells in eliminating cancer cells is one of the major factors fueling the growth of CAR-T cell therapy market in the country.
The ability of CAR-T cells to combat even highly resistant and recurring cancers that do not respond well to traditional therapies is driving its increased adoption. As South Korean cancer patients and their physicians get more exposure to successful CAR-T cell therapy outcomes globally, its demand is expected to grow significantly.
Market Driver – Robust Pipeline of CAR-T Cell Therapy Candidates
For instance, Anthropic is developing CAR-T therapies for acute lymphoblastic leukemia and lymphoma through genetic engineering techniques like self-supervision. Their trials have shown enhanced cell viability and stronger anti-tumor effects compared to first-generation CAR-T therapies. Similarly, Huono Therapeutics is conducting Phase 1/2 trials of an anti-BCMA CAR-T therapy for multiple myeloma patients who have failed other lines of treatment. Early results demonstrate high response rates with a favorable safety profile.
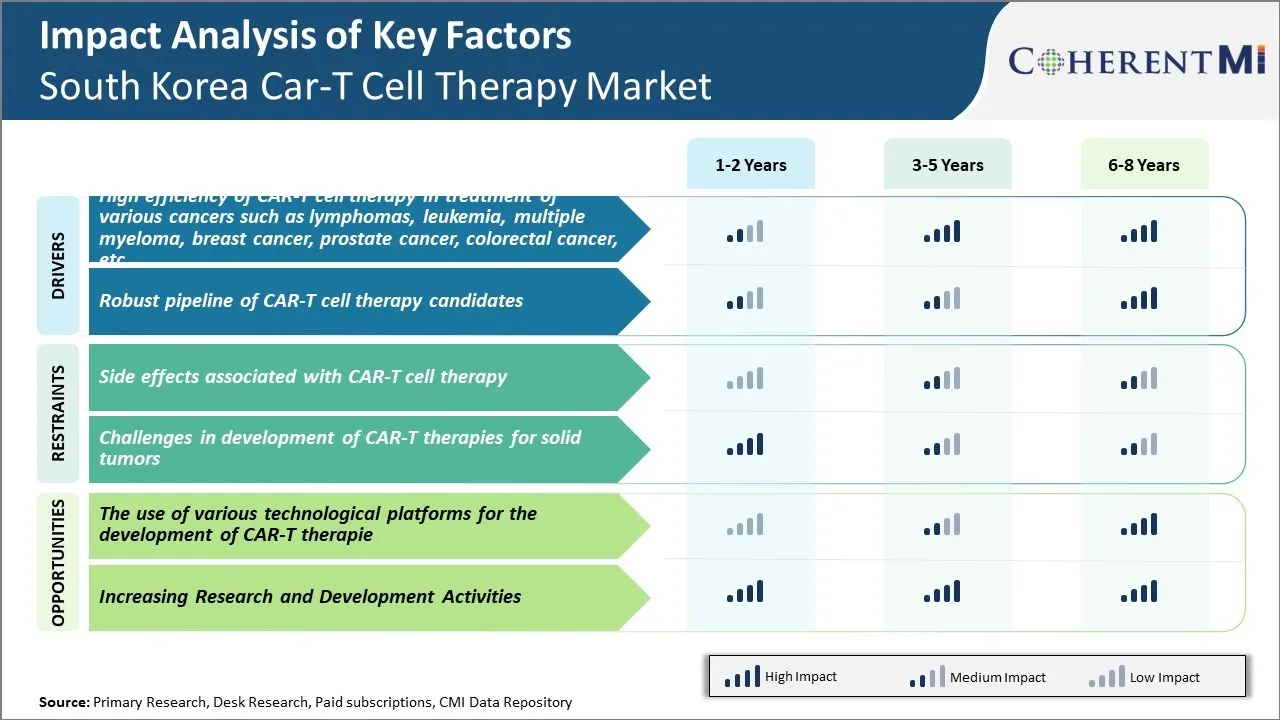
Market Challenge – Side Effects Associated with CAR-T Cell Therapy
The risks and uncertainties associated with CAR-T cell therapy side effects are making both doctors and patients hesitant to adopt this treatment approach. According to a 2020 study conducted by the Korea Centers for Disease Control and Prevention, out of the 44 adults who received CAR-T cell therapy for blood cancer at various hospitals in South Korea between 2017 to 2019, serious side effects were reported in 27 patients.
Market Opportunity – The Use of Various Technological Platforms for the Development of CAR-T Therapies
Several universities in South Korea are exploring new techniques for engineering CAR-T cells. For example, researchers at Korea University are developing novel gene editing platforms using CRISPR/Cas9 technology to improve the targeting ability and safety of CAR-T therapies. Preliminary research suggests their CRISPR-modified CAR-T cells show enhanced cancer cell killing ability with reduced off-target toxicity in animal models. Continued research on cutting-edge gene engineering methods could enable South Korean companies to design more effective CAR-T therapies. Additionally, Korean scientists are experimenting with innovative delivery vehicles for CAR-T cells. For instance, a team at Ulsan National Institute of Science and Technology has demonstrated the potential of an injectable hydrogel to protect CAR-T cells and allow for sustained release at tumor sites in preclinical trials.
Segmental Analysis of South Korea Car-T Cell Therapy Market
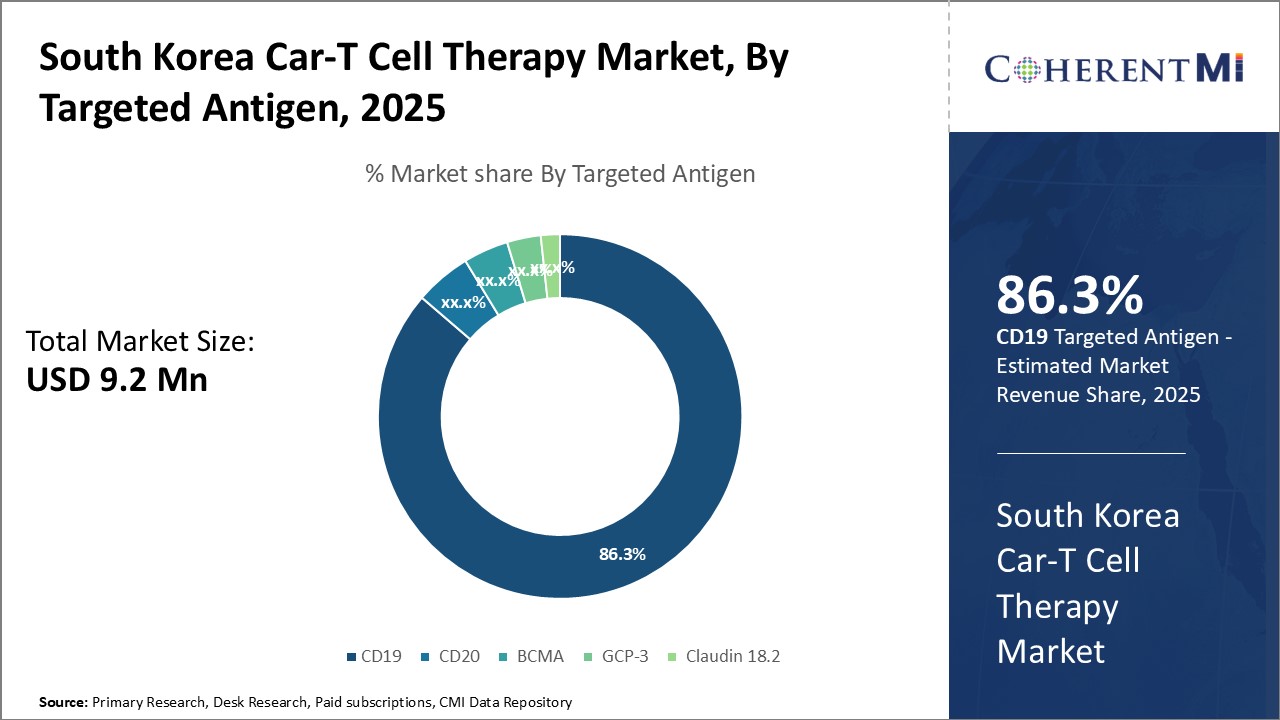 Insights, By Targeted Antigen: CD19 Dominates Targeted Antigens Due To Widespread Applicability In Lymphoma
Insights, By Targeted Antigen: CD19 Dominates Targeted Antigens Due To Widespread Applicability In Lymphoma Within the targeted antigen segments of the South Korea Car-T cell therapy market, CD19 sub-segment contributes the highest share of 86.3%. This is owing to its widespread applicability in treating B-cell malignancies such as non-Hodgkin's lymphoma. CD19 is expressed on B-cells and B-cell tumors, making it an ideal target for CAR-T therapies against B-cell lymphoma. Yescarta and Kymriah, two leading CAR-T therapies, target CD19 and have proven highly effective in clinical trials against lymphoma subtypes.
Additionally, CD19 CAR-T therapies have a relatively stable risk-benefit profile compared to other targets that are still under evaluation. Adverse effects are mostly manageable with modern protocols. This ease of use strengthens CD19 CAR-T uptake by both physicians and patients. Ongoing clinical research also continues expanding approved areas like third-line plus treatment and consolidating therapy.
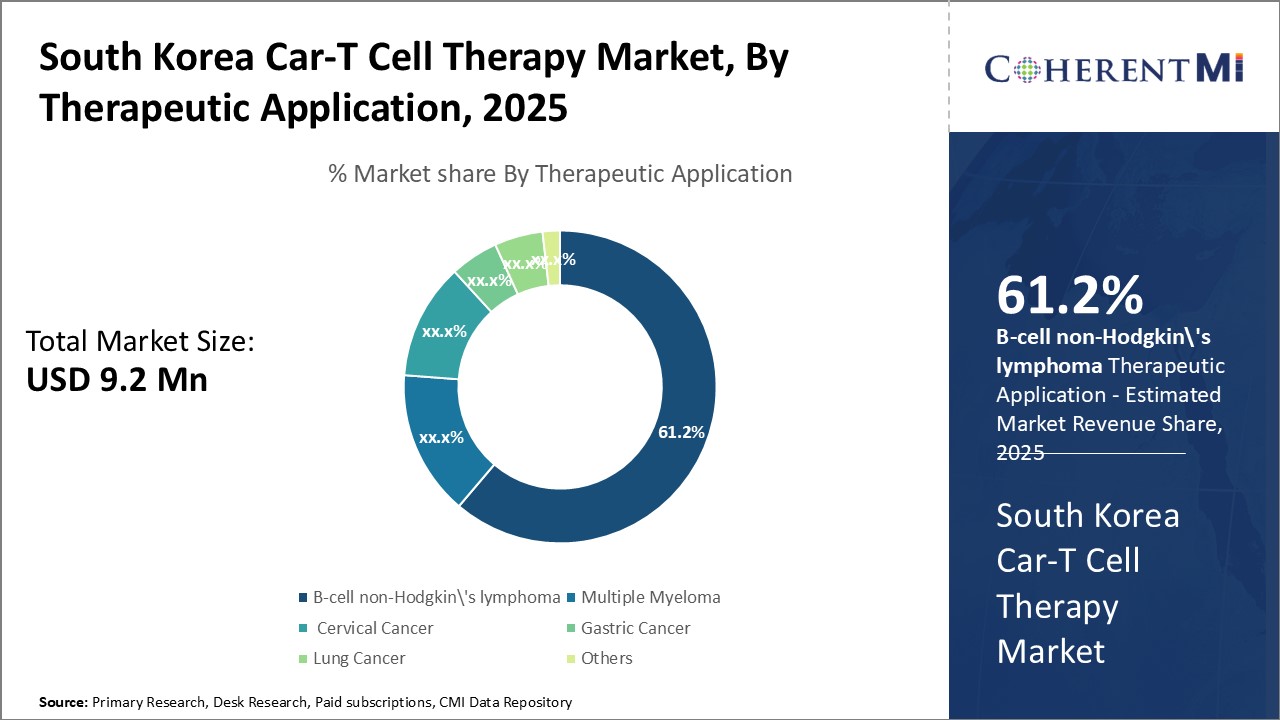
Within the therapeutic application segments of the South Korea CAR-T cell therapy market, B-cell non-Hodgkin's lymphoma sub-segment accounts for the highest share of 61.2%. This stems from non-Hodgkin's lymphoma posing a substantial disease burden in the country. South Korean government statistics indicate lymphoma as the tenth most prevalent cancer. Among lymphoma subtypes, diffuse large B-cell and follicular lymphoma have high incidence rates.
Despite response rates over 50% in clinical trials, non-Hodgkin's lymphoma remains difficult to cure, especially later stages. Traditional chemotherapy and immunotherapy have limitations such as cumulative toxicity and resistance. CAR-T cell therapy represents a major advance by tapping the immune system's own T-cells to produce durable remissions over 90% in some patients. This superior efficacy is driving oncologists’ commitment and interest to deploy CAR-T as the next frontier in lymphoma treatment.
Competitive overview of South Korea Car-T Cell Therapy Market
The major players operating in the South Korea Car-T Cell Therapy Market include Novartis AG, Pfizer, Inc., Bristol Myers Squibb, CARsgen Therapeutics Co., Ltd., Fate Therapeutics, Inc., Poseida Therapeutics, Inc., Eureka Therapeutics, Inc., Johnson & Johnson, Cellular Biomedicine Group, and Gilead Sciences, Inc.
South Korea Car-T Cell Therapy Market Leaders
- Novartis AG
- Pfizer, Inc.
- Bristol Myers Squibb
- CARsgen Therapeutics Co., Ltd.
- Fate Therapeutics, Inc.
South Korea Car-T Cell Therapy Market - Competitive Rivalry

South Korea Car-T Cell Therapy Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in South Korea Car-T Cell Therapy Market
- Celgene, a part of the U.S.-based pharmaceutical company called Bristol Myers Squibb, is conducting Phase III clinical trials (initiated on October 23, 2018) of its drug candidate JCAR017, a CD19-targeted chimeric antigen receptor (CAR) T-cell therapy indicated for the treatment of adults with relapsed or refractory (R/R) aggressive Non-Hodgkin lymphoma (NHL). This clinical trial study is expected to be completed by December 8, 2023.
- Novartis AG, a Switzerland-based pharmaceutical company, is conducting Phase III clinical trials (initiated on May 7, 2019) to evaluate the efficacy, safety, and tolerability of Tisagenlecleucel, a CAR-T cell therapy, as compared to standard of care in adult patients with aggressive B-cell Non-Hodgkin Lymphoma after failure of anthracycline and rituximab immunochemotherapy. This clinical trial study is expected to be completed by October 27, 2026.
- In June 2021, Blackstone, a leading global investment business, committed to an investment of US$ 250 million towards the launch of a new autologous and allogeneic universal chimeric antigen receptor (CAR) T-cell therapy company
- In June 2022, Bristol Myers Squibb announced that the U.S. Food and Drug Administration had approved Breyanzi (lisocabtagene maraleucel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, for the treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B
- In April 2022, Oncolytics Biotech Inc., a company that develops oncolytic viruses as potential therapeutics for use in a broad range of cancers, announced the publication of preclinical data demonstrating the synergistic anti-cancer activity of pelareorep combined with chimeric antigen receptor (CAR) T cell therapy in solid tumors. Combining CAR-T cells with pelareorep prevented antigen escape by creating CAR-T cells with dual specificity through a novel mechanism.
South Korea Car-T Cell Therapy Market Segmentation
- By Targeted Antigen
- CD19
- CD20
- BCMA
- GCP-3
- Claudin 18.2
- By Therapeutic Application
- B-cell non-Hodgkin's lymphoma
- Multiple Myeloma
- Cervical Cancer
- Gastric Cancer
- Lung Cancer
- Others

Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Frequently Asked Questions :
How big is the South Korea Car-T Cell Therapy Market?
The South Korea Car-T Cell Therapy Market is estimated to be valued at USD 9.2 in 2025 and is expected to reach USD 26.5 Million by 2032.
What are the major factors driving the South Korea Car-T Cell Therapy Market growth?
The high efficiency of Car-T cell therapy in treatment of various cancers such as lymphomas, leukemia, multiple myeloma, breast cancer, prostate cancer, colorectal cancer, etc. and robust pipeline of Car-T cell therapy candidates are the major factors driving the South Korea Car-T Cell Therapy Market growth.
Which is the leading Targeted Antigen in the South Korea Car-T Cell Therapy Market?
The leading Targeted Antigen segment is CD19.
Which are the major players operating in the South Korea Car-T Cell Therapy Market?
Novartis AG, Pfizer, Inc., Bristol Myers Squibb, CARsgen Therapeutics Co., Ltd., Fate Therapeutics, Inc., Poseida Therapeutics, Inc., Eureka Therapeutics, Inc., Johnson & Johnson, Cellular Biomedicine Group, and Gilead Sciences, Inc. are the major players.
What will be the CAGR of the South Korea Car-T Cell Therapy Market?
The CAGR of the South Korea Car-T Cell Therapy Market is projected to be 16.3% from 2025-2032.