Acute Radiation Syndrom Market GRÖSSEN- UND MARKTANTEILSANALYSE - WACHSTUMSTRENDS UND PROGNOSEN (2024 - 2031)
Acute Radiation Syndrom Market wird durch Strahlungsart (Ionizing Radiation, Non-ionizing Radiation), By Product Type (Kapseln, Tabletten, Parenterals....
Acute Radiation Syndrom Market Größe
Marktgröße in USD Bn
CAGR5%
| Studienzeitraum | 2024 - 2031 |
| Basisjahr der Schätzung | 2023 |
| CAGR | 5% |
| Marktkonzentration | High |
| Wichtige Akteure | Statera BioPharma, NeoImmuneTech, Zellstofftherapie, Partner-Therapeutik, Pluristem Therapeutics und unter anderem |
Bitte lassen Sie es uns wissen!
Acute Radiation Syndrom Market Analyse
Das Global Acute Radiation Syndrom Der Markt wird geschätzt USD 5.2 Bn in 2024 und wird voraussichtlich erreichen USD 7,3 Bn bis 2031, Wachstumsrate (CAGR) von 5 % von 2024 bis 2031. Die zunehmende Prävalenz von Krebsfällen, die durch veränderte Lebensgewohnheiten und übermäßige Strahlenexposition verursacht werden, hat die Nachfrage nach akuten Strahlensyndrombehandlungen im Laufe der Jahre erhöht.
Das Akute-Strahlungs-Syndrom (ARS), auch als Strahlenkrankheit bezeichnet, tritt nach Einwirkung hoher Dosen ionisierender Strahlung über einen kurzen Zeitraum auf. Es wirkt sich auf schnell teilende Zellen im Körper, insbesondere im Knochenmark, Magen-Darm-Trakt und Zentralnervensystem. Symptome reichen von Übelkeit und Müdigkeit bis zu schweren Organschäden und Tod, je nach Strahlendosis. Sofortige medizinische Eingriffe sind entscheidend für das Überleben, einschließlich Behandlungen zur Erhöhung der Immunfunktion und zur Verringerung der Strahlenschäden. Der Markt erlebt im Prognosezeitraum positive Wachstumstrends. Fortschritte in der Strahlen-Onkologie für eine effektive Krebsbehandlung mit Strahlentherapie zusammen mit der wachsenden Zulassung und Annahme neuartiger Therapeutika für akutes Strahlensyndrom werden erwartet, den Markt voranzutreiben. Darüber hinaus wird erwartet, dass die zunehmende Investition durch Schlüsselakteure für die Entwicklung innovativer Behandlungsoptionen auch das Marktwachstum in dieser Zeit unterstützen wird.
Acute Radiation Syndrom Market Trends
Markttreiber - Erhöhung globaler Belange über nukleare Emergenzen und Strahlenexposition treiben die Nachfrage nach Behandlungen.
Da globale Ereignisse den Menschen die Gefahren und gesundheitlichen Risiken, die durch nukleare Notfälle und Strahlenexposition entstehen, stärker bewusst gemacht haben, sind Fortschritte bei medizinischen Gegenmaßnahmen zur Behandlung des akuten Strahlensyndroms immer wichtiger geworden. Jeder nukleare Notfall, sei es aufgrund von Unfällen, Abschmelzen oder Angriffen, könnte möglicherweise große Populationen auf gefährliche Strahlungsniveaus aussetzen. Während ständig Anstrengungen unternommen werden, um Sicherheit und Sicherheit weltweit zu verbessern, können die Risiken solcher Katastrophen niemals vollständig beseitigt werden. Infolge von hochkarätigen Ereignissen in den letzten Jahrzehnten wie dem Aufschmelzen von Tschernobyl- und Fukushima-Kernkraftwerken sowie der Verschärfung von Besorgnissen über die Möglichkeit des Nuklearterrorismus hat das öffentliche Bewusstsein für strahlungsgefährdete Bedrohungen weltweit beispiellose Ausmaße erreicht. Die Menschen sind nun mehr über akute Strahlenkrankheit und ihre potentiellen Symptome wie Übelkeit, Erbrechen, Durchfall, Hautverbrennungen und Verletzungen von inneren Organen aus sehr hohen Strahlenexposition. Die potentielle Skala von Opfern aus einem groß angelegten Ereignis bedeutet, dass der Fokus auf die Entwicklung medizinischer Reaktionen, die Strahlenkrankheit über ganze Populationen in jedem betroffenen Bereich effektiv behandeln kann, erhöht wird. Pharmazeutische Unternehmen und Forscher erkennen diese wachsende Marktchance und müssen gezieltere und wirksamere Behandlungsprotokolle, diagnostische Werkzeuge und therapeutische Medikamente zur Strahlenverletzung voranbringen. Dieses Klima der Besorgnis über nukleare/strahlungsbedingte Notfälle und die Aussicht auf die Unterstützung medizinischer Organisationen bei der Vorbereitung auf potenzielle Todesfälle in Massenvernichtungsfällen führt dazu, dass in diesem Sektor größere Investitionen und F&D gefördert werden.
Markttreiber - Fortschritte in biologischen Therapien und Strahlung Gegenmaßnahmen bieten neue Avenues für die Behandlung.
Wissenschaftlicher Fortschritt eröffnet nach und nach vielversprechende neue Ansätze zur Bekämpfung der akuten Strahlenkrankheit. Fortgeschrittene Untersuchungen zur Zell- und Molekularbiologie haben zu einem tieferen Verständnis geführt, wie Strahlung Gewebe auf mikroskopischen Ebenen schädigt und die komplexen biochemischen Wege des Körpers stört. Mit diesem erweiterten Wissen haben Forscher neuartige Biotherapien entwickelt, die natürliche Mechanismen zur Unterstützung der Wiederherstellung von Strahlenschäden nutzen. Zelltherapien mit mesenchymalen Stammzellen zeigen die Fähigkeit, die Geweberekonstruktion zu beschleunigen und Entzündungen in Tierstudien zu reduzieren. Auch Wachstumsfaktoren und Zytokine werden als Mittel zur Regeneration der Zellen nach Strahlenschäden untersucht. Gene Therapietechniken ermöglichen eine gezielte Lieferung von Genen, die für Schutz-, Reparatur- oder entzündungshemmende Proteine kodieren. Auf der pharmazeutischen Front sind neue Drogenanwärter für Strahlenverletzungen wie Amyloid-Inhibitoren und Anti-Nausea-Medikamente in der Entwicklung. Kombinationstherapien mit mehreren Behandlungsmodalitäten zeigen auch potenzielle Synergien. Alle diese innovativen therapeutischen Alleen zielen darauf ab, Zellen vorab zu schützen, Strahlenvergiftungssymptome akut zu mildern oder die Heilung des Körpers zu steigern, sobald die Exposition auftritt. Mit fortwährenden Fortschritten könnten sie irgendwann im Vergleich zu herkömmlichen Ansätzen eine weitaus effektivere medizinische Behandlung von Strahlenunfällen übersetzen. Diese aufstrebende Reihe von biologischen und molekularen Gegenmaßnahmen bietet somit viel Hoffnung, die laufende FuE-Unterstützung und die Erwartungen an die Pflege voranzutreiben.
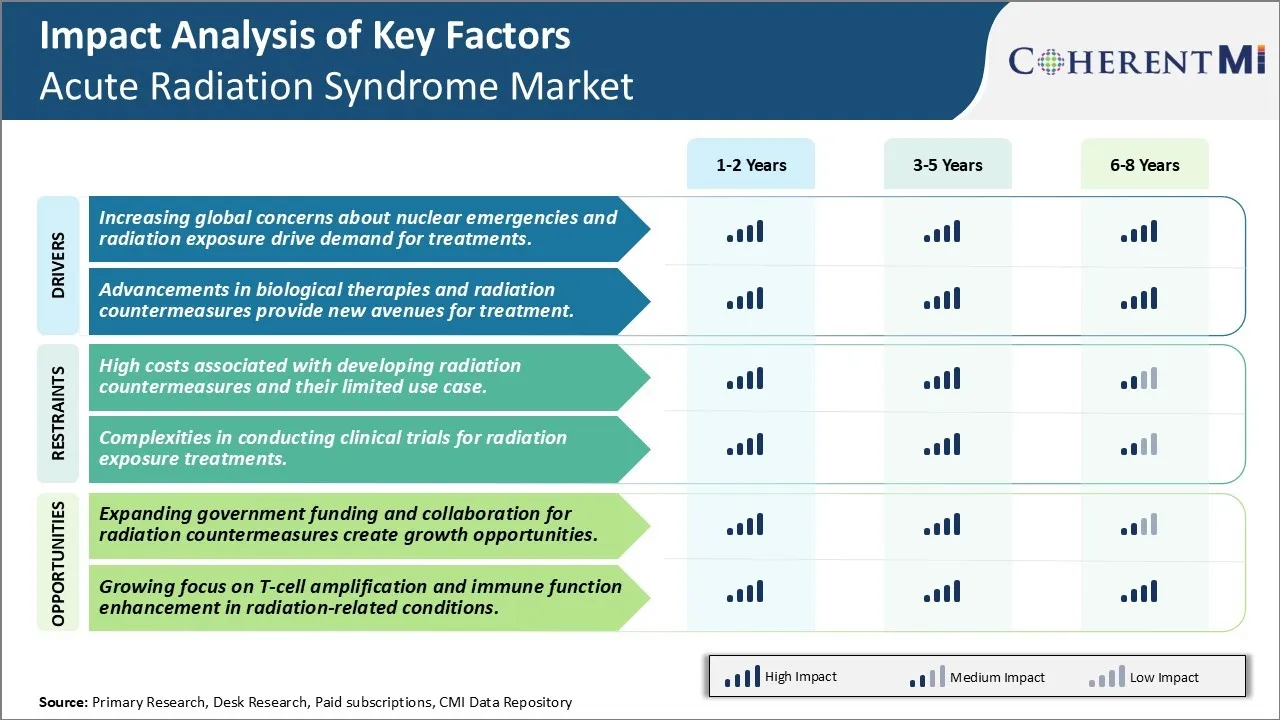
Markt-Herausforderung - Hohe Kosten verbunden mit der Entwicklung von Strahlung Gegenmaßnahmen und ihre begrenzte Verwendung Fall.
Eine der wichtigsten Herausforderungen für den akuten Strahlungsssyndrommarkt sind die hohen Kosten, die mit der Entwicklung von Strahlungsgegenmaßnahmen und ihrem begrenzten Anwendungsfall verbunden sind. Die Entwicklung wirksamer Gegenmaßnahmen erfordert umfangreiche Forschung und Tests, die über mehrere Jahre hohe finanzielle Investitionen beinhalten. Da Strahlungsnotfälle relativ seltene Ereignisse sind, ist auch die potentielle Kundenbasis für diese Produkte recht begrenzt. Dies begrenzt die kommerzielle Rentabilität und die Rendite von Investitionen für Unternehmen auf diesem Markt. Darüber hinaus, da akutes Strahlensyndrom nur unter extremen Umständen wie Atomunfälle oder Angriffe auftritt, ist die Nachfrage unvorhersehbar und lumpig in der Natur. Die Notwendigkeit dieser Gegenmaßnahmen hängt auch von geopolitischen Risiken und Sicherheitssituationen in Regionen ab. All diese Faktoren machen es für Unternehmen sehr schwierig, die hohen Kosten für Forschung und Entwicklung für Strahlungsgegenmaßnahmen zu rechtfertigen. Die Auszahlungen sind unsicher und die Kosten sind weitgehend nicht wiedererfassbar, wenn die Produkte nicht kommerziell genutzt werden. Dies wirkt als Abschreckung für Investitionen im privaten Sektor in diesem Segment.
Marktchancen - Ausweitung der staatlichen Förderung und Zusammenarbeit für Strahlungszähler Maßnahmen schaffen Wachstumschancen.
Der akute Strahlungsssyndrommarkt wird durch die Ausweitung der staatlichen Fördermittel und die Zusammenarbeit bei der Forschung in Strahlungsgegenmaßnahmen mit mehreren Wachstumschancen präsentiert. Viele Regierungen und globale Institutionen erkennen die Bedeutung der Entwicklung wirksamer medizinischer Reaktionen auf Strahlungsnotfälle an. Sie unterstützen die Forschung durch verstärkte öffentliche Finanzierung und Partnerschaften. So investiert die US-Regierung stark in die Entwicklung medizinischer Gegenmaßnahmen durch die Biomedizinische Advanced Research and Development Authority. Ebenso finanziert die Europäische Kommission Projekte, die die medizinische Bereitschaft verbessern sollen. Diese stabile Finanzierungspipeline reduziert Investitionsrisiken für Unternehmen. Darüber hinaus kommen branchenübergreifende Kooperationen und Konsortiummodelle zum Ziel, Ressourcen zu vereinen und Kosten und Risiken zu verbreiten, die mit der Forschung verbunden sind. Solche Initiativen haben mehrere neue Produktpipeline-Programme angestoßen. Sie ziehen auch die Teilnahme von mehr Spielern an. Die kollektiven Anstrengungen sollen die Produktentwicklung und die Vermarktung beschleunigen und den Markt vorantreiben.
Präferenzen der Verschreiber von Acute Radiation Syndrom Market
Akute Radiation Syndrom (ARS) wird in der Regel in 3 Stufen behandelt - die prodromalen, latenten und Erholungsstadien - je nach Schwere der Strahlenexposition. Im prodromalen Stadium mit milder Strahlungsexposition (<2Gy) entscheiden sich die Beschreiber im Allgemeinen für eine unterstützende Pflege durch Hydratation und Antiemetik wie Ondansetron, um Übelkeit und Erbrechen zu verwalten.
Bei Patienten mit moderaten Strahlungspegeln (2-6Gy) ist die latente Stufe eine verstärkte CBC-Überwachung sowie eine Behandlung mit Neupogen (filgrastim) zur Erhöhung der Neutrophilzahl erforderlich. Wenn CBC-Spiegel weiter sinken, können Verschreibungen auch Antibiotika wie Levaquin (levofloxacin) hinzufügen, um Infektionen zu verhindern.
Bei starker Strahlenexposition (>6Gy) konzentriert sich die Regenerationsphase auf eine intensive CBC-Unterstützung zusammen mit Breitspektrum-Antibiotika, um Sepsis zu verhindern. Schreiber wählen häufig zwischen Neupogen und Leukine (sargramostim) für hämatopoietische Wachstumsfaktorunterstützung. Blutprodukttransfusionen sind auch weit verbreitet, um verlorene Zelllinien zu ersetzen.
Zusätzlich führen die individuelle Strahlendosis eines Patienten, die Einbeziehung des Organsystems, die vorhandenen Bedingungen und die Zeitverzögerung, da die Exposition auch die Beschreiber bei der Auswahl des am besten geeigneten Behandlungsansatzes, der Dosierung und der Dauer von Fall zu Fall führen, um die Ergebnisse für ARS-Patienten zu maximieren. Eine enge Überwachung der Nebenwirkungen hilft weiter, die Therapie zu optimieren.
Analyse der Behandlungsoptionen von Acute Radiation Syndrom Market
Akute Strahlungsssyndrom (ARS) kann in vier primäre Stufen auf Basis von Symptomen und Prognosen unterteilt werden - mild, moderat, schwer und akut. Die Behandlung hängt von der Phase des ARS ab.
In milden Fällen kann eine unterstützende Pflege einschließlich Flüssigkeiten und Antiemetik ausreichend sein. Für moderate ARS werden häufig Wachstumsfaktoren wie G-CSF oder GM-CSF eingesetzt. Diese stimulieren die Knochenmarkaktivität und reduzieren das Infektionsrisiko.
Bei schweren ARS-Fällen kann die Knochenmarktransplantation berücksichtigt werden, insbesondere wenn das gesamte Knochenmark hohen Strahlendosen ausgesetzt war. Die Transplantation ergänzt das Immunsystem. Zur Transplantation verwendete übliche Medikamente sind Cyclophosphamid und die Gesamtkörperbestrahlung als Konditionierung. Passende Stammzellen aus einem Spender werden dann infundiert.
Die kritischste Phase ist die akute ARS, wo Multi-Organ-Versagen unmittelbar ohne Eingriff. Die Behandlung der Wahl ist unterstützende Pflege zusammen mit Wachstumsfaktoren, um verbleibende Knochenmark zu stimulieren. Wenn der Patient stabil genug ist, bietet eine Knochenmarktransplantation die beste Überlebenschance. Alternativ werden wegweisende Behandlungen mit Stammzellen, die direkt an verletzte Gewebe geliefert werden, untersucht, da sie die Funktion schneller wiederherstellen können als herkömmliche Transplantation.
Zusammenfassend hängt die Behandlung von der ARS-Stufe ab, von der unterstützenden Pflege bis zu Wachstumsfaktoren bis zur Transplantation. Die gezielte Ausrichtung der zugrunde liegenden Knochenmarkschaden und die Wiederherstellung von Hämatopoies ist entscheidend, da ARS die Fähigkeit des Körpers beeinträchtigt, infektiöse Blutzellen zu produzieren. Stammzelltherapien zeigen Versprechen, aber Transplantate bleiben die Standardbehandlung derzeit, wenn möglich.
Wichtige Erfolgsstrategien der Hauptakteure von Acute Radiation Syndrom Market
Partnerschaften und Kooperationen zur Drogenentwicklung: Im Januar 2024 arbeitete BARDA (Biomedical Advanced Research and Development Authority) mit Partner Therapeutics zusammen, um Leukine als Behandlungslösung für ARS zu entwickeln und zu erweitern. Die Partnerschaft stärkt Gesundheitsinitiativen durch einen besseren Zugang zu effektiven ARS-Behandlungen in Notsituationen.
Geographische Expansion in Regierungsverträge: 2024 erweiterte Partner Therapeutics seine Verbreitung von Leukine® (sargramostim), einem zur Behandlung von ARS verwendeten Immunmodulator, indem er einen Regierungsvertrag mit Kanada abschließt. Der Vertrag gewährleistet die Verfügbarkeit von Leukine® für nationale Lagerbestände in Vorbereitung auf potenzielle nukleare oder radiologische Notfälle. LeLukine® wurde bereits in den USA unter Regierungsverträgen mit der Biomedical Advanced Research and Development Authority (BARDA) eingesetzt, und diese Expansion in Kanada stellt ein bedeutendes geografisches Wachstum für Partner Therapeutics in Bezug auf die Regierung dar.
Fokus auf Forschung und Entwicklung fortgeschrittener Behandlungsoptionen: Im Jahr 2023 hat Pluristem Therapeutics PLX-R18 entwickelt, die die Regeneration von Knochenmark und die Wiederherstellung der Blutzellproduktion nach Strahlenexposition fördert.
Die vorstehenden Beispiele illustrieren, wie strategische Investitionen in FuE, Kooperationen zur Drogenentwicklung und geografische Expansion in Regierungsverträge Führungspersönlichkeiten wie Partner Therapeutics, Pluristem Therapeutics anderen geholfen haben, führende Positionen im akuten Strahlensyndrommarkt zu gewinnen. Ihr Fokus auf fortschrittliche Behandlungslösungen und die Fähigkeit, Finanzierung zu sichern, hat weitere Marktentwicklungen vorangetrieben.
Segmentanalyse von Acute Radiation Syndrom Market

Insights, By Radiation Type, Rising Usage of X-rays and Radiation Therapy Drives Ionizing Radiation Segment.
Durch die Ionisationsart trägt ionisierende Strahlung 2024 aufgrund der zunehmenden Nutzung von Röntgen- und Strahlentherapie für medizinische Diagnose- und Behandlungszwecke den höchsten Anteil von 68,2% bei. Ionisierende Strahlung wie Röntgenstrahlen und Gammastrahlen haben Energie hoch genug, um eng gebundene Elektronen aus Atomen zu entfernen, ionisierend. Diese Art von Strahlung wird häufig in Praktiken wie diagnostische Röntgenbildgebung, CT-Scanning, nuklearmedizinische Bildgebung und Strahlentherapie für die Krebsbehandlung verwendet.
Die wachsende geriatrische Bevölkerung anfällig für Krankheiten wie Krebs hat die Nachfrage nach diagnostischen Röntgenuntersuchungen und Strahlentherapie-Sessions in den letzten Jahren deutlich erhöht. Die Strahlentherapie ist eine Standardbehandlungsoption, entweder allein oder in Kombination mit Chirurgie oder Chemotherapie für zahlreiche Krebsarten. Fortschritte in der Strahlentherapie-Techniken, die eine höhere Präzision und lokalisierte Dosisabgabe ermöglichen, haben auch seine Verwendung erweitert. Darüber hinaus ist das Bewusstsein für die Wirksamkeit der Frühkrebserkennung durch diagnostische Bildgebung dazu führen, dass mehr Menschen sich für Routine-Volumen-Röntgen-Scans entscheiden.
Allerdings erhöht die Exposition gegenüber ionisierender Strahlung auch auf medizinisch bedingten Ebenen das Risiko eines akuten Strahlungssyndroms durch Schädigung von Zellen und Geweben. Häufige Symptome sind Übelkeit, Erbrechen, Durchfall, Hautverbrennungen und Müdigkeit. Eine längere oder hohe Dosisbelastung kann in schweren Fällen zu Organschäden, Blutungen, Unfruchtbarkeit und sogar zum Tod führen. Dies hat die Notwendigkeit wirksamer Gegenmaßnahmen zur Behandlung von Strahlungsvergiftungen durch diagnostische und therapeutische ionisierende Strahlungsverfahren verstärkt. Die große Patientenbasis, die solche Verfahren regelmäßig durchläuft, macht ionisierende Strahlung zum dominanten Segment im akuten Strahlensyndrommarkt.
Insights, Nach Produkttyp, Popularität von Capsules Drives Höchster Anteil der Akute Strahlungs-Syndrom Behandlung.
Unter verschiedenen Produkttypen tragen Kapseln aufgrund ihrer Popularität und weit verbreiteten Akzeptanz als bevorzugte orale Darreichungsform den höchsten Anteil 48,3% in 2024 bei. Kapseln bieten Vielseitigkeit in Dosierung und einfache Verabreichung im Vergleich zu anderen oralen Optionen wie Tabletten und Flüssigkeiten. Sie bieten eine Reihe von Formulierungsvorteilen, die ihre Top-Position auf dem Markt verklebt haben.
Kapseln erlauben die Unterbringung eines breiten Spektrums von Wirkstoffen - von kleinen Moleküldrogen bis zu großen Biomolekülen. Ihre Gelatine-Außenschale schützt empfindliche Verbindungen und ermöglicht die enterische Freisetzung an bestimmten Stellen im Magen-Darm-Trakt. Dies macht Kapseln geeignet für Medikamente, die Schutz vor Magensäure oder Targeting des unteren Darms benötigen. Das leere Innere der Kapseln bietet auch die Flexibilität, Kombinationen von Pulvern, Granulaten und Flüssigkeiten in eine einzige Dosierungsform zu laden.
Kapseln lassen sich anhand ihrer Form, Farbe und Aufdrucke im Vergleich zu unbeschichteten Tabletten leicht identifizieren. Sie benötigen kein Wasser für Ingestion und sind weniger anfällig für Risiken bei jungen, älteren oder unbewussten Patienten. Kapselschalen bieten eine zusätzliche Sicherheitsschicht für eine versehentliche Aufnahme durch junge Kinder im Vergleich zu lose Pulver oder sogar Tabletten. Alle diese Vorteile gegenüber Alternativen wie parenteralen Injektionen haben Kapseln als bevorzugtes Lieferfahrzeug für orale akute Strahlensyndrombehandlungen etabliert.
Zusätzliche Einblicke von Acute Radiation Syndrom Market
Akute Radiation Syndrom (ARS) bleibt ein bedeutendes Anliegen der öffentlichen Gesundheit, insbesondere im Kontext potenzieller nuklearer Notfälle. Der Zustand ist durch schwere Schäden an Organsystemen gekennzeichnet, die oft zu tödlichen Ergebnissen ohne sofortige Intervention führen. STAT-600 von Statera BioPharma stellt eine wichtige Weiterentwicklung der Strahlungsgegenmaßnahmen dar, indem mühsame Rezeptoren aktiviert werden, die die Immunfunktion verbessern und die Auswirkungen der Strahlung mildern können. Ähnlich, NeoImmune Tech's NT-I7 zeigt Versprechen in präklinischen Studien durch Verstärkung von T-Zellen und bietet einen potenziellen immunmodulatorischen Ansatz zur Behandlung von Strahlensyndrom. Die laufende Entwicklung dieser Therapien unterstreicht den dringenden Bedarf an neuartigen Ansätzen zur Behandlung von ARS, insbesondere angesichts der Einschränkungen aktueller Behandlungen. Mit einer verstärkten staatlichen Unterstützung und Zusammenarbeit zwischen akademischen und privaten Institutionen wird die ARS-Behandlungslandschaft in den kommenden Jahren für ein erhebliches Wachstum gesichert. Fortschritte bei der Immuntherapie und gezielten biologischen Behandlungen können nicht nur Leben retten, sondern auch die Qualität der Pflege für Personen verbessern, die hohen Strahlendosen ausgesetzt sind.
Wettbewerbsübersicht von Acute Radiation Syndrom Market
Zu den wichtigsten Akteuren im Acute Radiation Syndrom Market gehören Statera BioPharma, NeoImmuneTech, Cellerant Therapeutics, Partner Therapeutics, Pluristem Therapeutics, Humanetics Corporation, Amgen, Recipharm AB, Mission Pharmacal Company, Partner Therapeutics, Novartis AG und Mylan NV.
Acute Radiation Syndrom Market Marktführer
- Statera BioPharma
- NeoImmuneTech
- Zellstofftherapie
- Partner-Therapeutik
- Pluristem Therapeutics
Acute Radiation Syndrom Market - Wettbewerbsrivalität

Acute Radiation Syndrom Market
(Von großen Akteuren dominiert)
(Hoher Wettbewerb mit vielen Akteuren.)
Neueste Entwicklungen in Acute Radiation Syndrom Market
- Im Mai 2024 erweiterte Statera BioPharma sein STAT-600 Medikament auf Phase-I-Tests, die Immunverbesserung als Gegenmaßnahme zum Strahlungsssyndrom anregen, indem es mühsame Rezeptoren zur Minderung von Strahlenschäden anregt.
- Im März 2024, NeoImmune Tech kündigte preklinische Ergebnisse für NT-I7 an, die Potenziale bei der Erhöhung der T-Zell-Verstärkung und der Wiederherstellung der Immunfunktionalität nach der Bestrahlung zeigten.
Acute Radiation Syndrom Market Segmentierung
- Durch Strahlungsart
- Ionisierende Strahlung
- Nichtionisierende Strahlung
- Nach Produkttyp
- Kapseln
- Tabletten
- Sparkassen
- Sonstige

Möchten Sie die Möglichkeit erkunden, einzelne Abschnitte dieses Berichts zu kaufen?
Häufig gestellte Fragen :
Wie groß ist der Acute Radiation Syndrom Market?
Der Global Acute Radiation Syndrom Market wird im Jahr 2024 auf USD 5,2 Mrd. geschätzt und wird voraussichtlich bis 2031 USD 7,3 Mrd. erreichen.
Was wird das CAGR des Acute Radiation Syndrom Market sein?
Der CAGR des Acute Radiation Syndrom Market wird von 2024-2031 auf 5% projiziert.
Was sind die Hauptfaktoren für das Wachstum des Acute Radiation Syndrome Market?
Die zunehmenden globalen Bedenken hinsichtlich der nuklearen Notfälle und der Nachfrage nach Strahlenbelastungen nach Behandlungen und Fortschritten in biologischen Therapien und Strahlungsgegenmaßnahmen stellen neue Wege zur Behandlung dar, sind die wichtigsten Faktoren, die den Acute Radiation Syndrom Market antreiben.
Was sind die wichtigsten Faktoren, die das Wachstum des Acute Radiation Syndrom Market behindern?
Die hohen Kosten, die mit der Entwicklung von Strahlungsgegenmaßnahmen verbunden sind, sowie deren begrenzte Anwendungsfälle und Komplexitäten bei der Durchführung von klinischen Studien zur Strahlenexpositionsbehandlung sind der Hauptfaktor, der das Wachstum des Acute Radiation Syndrom Markets behindert.
Welches ist der führende Strahlentyp im Akute-Strahlungs-Syndrommarkt?
Ionizing Radiation ist das führende Radiation Type Segment.
Welche sind die Hauptakteure im Acute Radiation Syndrom Market?
Statera BioPharma, NeoImmuneTech, Cellerant Therapeutics, Partner Therapeutics, Pluristem Therapeutics, Humanetics Corporation, Amgen, Recipharm AB, Mission Pharmacal Company, Partner Therapeutics, Novartis AG, Mylan NV sind die wichtigsten Akteure.