Acid Sphingomyelinase Deficiency (ASMD) Market Trends
Market Driver - Growing Prevalence of ASMD, Coupled with Increasing Awareness and Diagnostics
ASMD is an extremely rare lysosomal storage disease caused by mutations in the SMPD1 gene which result in a deficiency of the acid sphingomyelinase enzyme. This genetic defect prevents the normal breakdown of sphingomyelin, a fatty substance found in membranes of cells, causing it to accumulate to harmful levels over time.
Historically, ASMD was considered one of the rarest lysosomal diseases with estimates of only around 50 to 150 known patients worldwide. However, new research indicates the actual prevalence may be higher than previously believed as improved diagnostics allow for more cases to be accurately identified. Additionally, it is now recognized that the condition exists in both a severe infantile form and a milder late-onset form.
As the true prevalence of ASMD comes into better focus, efforts are ongoing to raise awareness about the condition among the medical community and public. Patient advocacy groups play an important role in educating about signs, symptoms and available testing options. Diagnosis traditionally relied on invasive bone marrow biopsy but new dry blood spot tests allow for more convenient newborn screening and diagnosis of late-onset patients. Increased overall awareness combined with easier diagnostic pathways is facilitating the identification and confirmation of more ASMD cases worldwide.
Market Driver - FDA Approval of XENPOZYME and its Strong Market Demand in the US and Europe
Another key driver within the ASMD treatment market is the FDA's recent approval of XENPOZYME, the first ever drug specifically indicated for this rare genetic disorder. Developed by Genzyme, a Sanofi company, XENPOZYME received accelerated approval in January 2022 based on data demonstrating its ability to reduce sphingomyelin accumulation in cells. It is a recombinant human acid sphingomyelinase enzyme replacement therapy administered weekly as an IV infusion or injection intended to replace the deficient or missing enzyme.
XENPOZYME represents the first ever disease-modifying treatment option for ASMD which was previously managed solely through symptomatic care. Clinicians recognize it has the potential to meaningfully impact the often-devastating multisystem complications of this progressive condition. Initial real-world experience supports the positive efficacy and safety demonstrated in clinical trials.
Market research focusing on both patient and physician surveys indicates XENPOZYME will likely become the standard of care treatment for ASMD worldwide. Overall, the availability of this groundbreaking targeted therapy is anticipated to be a major growth driver, increasing diagnosis rates and fueling the overall acid sphingomyelinase deficiency (ASMD) market value in the coming years.
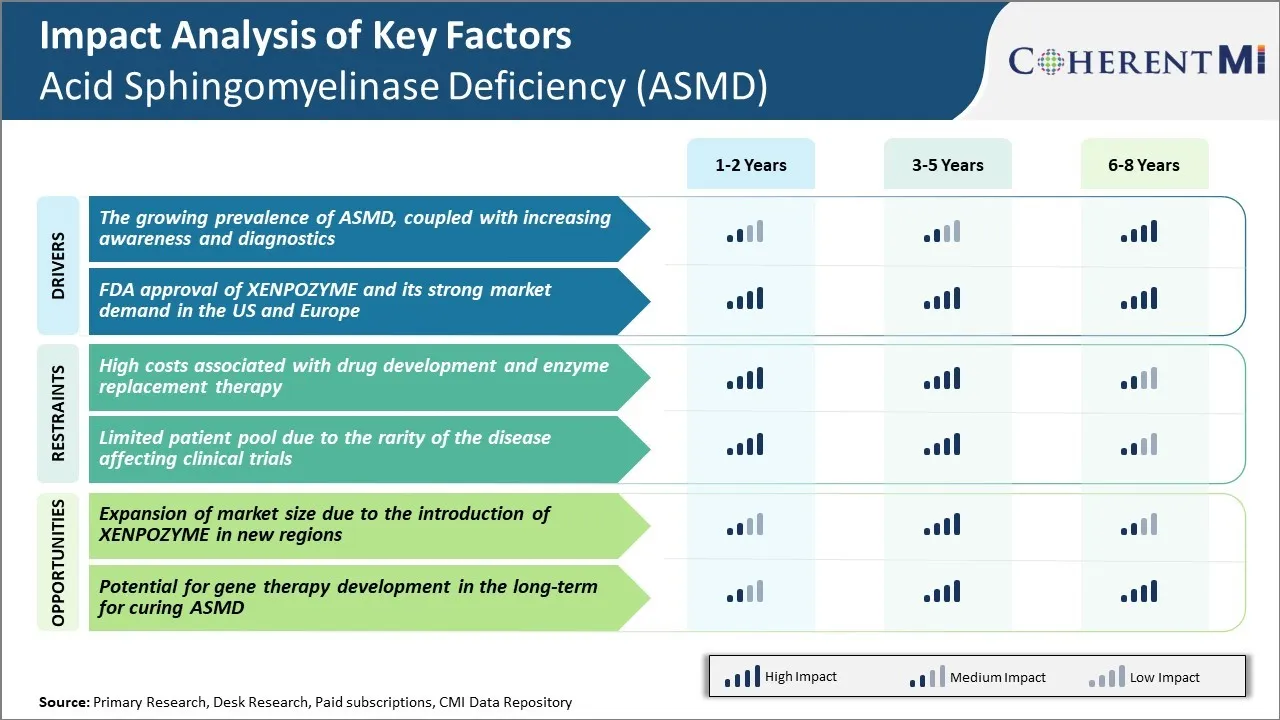
Market Challenge - High Costs Associated with Drug Development and Enzyme Replacement Therapy
The acid sphingomyelinase deficiency (ASMD) market faces significant challenges due to the high costs associated with drug development and enzyme replacement therapy for this rare disease. Developing drugs to treat such a rare condition requires extensive research and clinical trials to demonstrate safety and efficacy in small patient populations. This drug development process is very expensive and risky for pharmaceutical companies to undertake.
Additionally, the approved enzyme replacement therapy called Cerdelga is priced at over $300,000 per year, making it too costly for many healthcare systems and patients. The small patient numbers mean the overall size of acid sphingomyelinase deficiency (ASMD) market is limited, providing little revenue potential for pharmaceutical companies to recoup their drug development investments.
High production costs are also incurred for enzyme replacement therapies due to their complex biologic nature. These significant financial challenges may discourage further research into new treatment options and limit patient access to existing therapies for ASMD.
Market Opportunity - Expansion of Market Size due to the Introduction of XENPOZYME in New Regions
The approval and commercialization of the new ASMD drug XENPOZYME represents a major opportunity to expand the market size for this condition. XENPOZYME, developed by Xenetic Biosciences, has demonstrated safety and efficacy in clinical trials.
With regulatory approval, the company is planning to launch XENPOZYME in the key markets of the United States, Europe and Japan in 2023. This will provide ASMD patients in these new regions with an important additional treatment option. The introduction of XENPOZYME also has the potential to significantly grow the overall acid sphingomyelinase deficiency (ASMD) market.
As XENPOZYME gains uptake among eligible patients, it could start to offset some of the market currently held by Cerdelga. This market expansion would help improve the return on investment for companies developing therapies for this rare disease population.