Acute Radiation Syndrome Market Size - Analysis
Acute Radiation Syndrome (ARS), also known as radiation sickness, occurs after exposure to high doses of ionizing radiation over a short period. It affects rapidly dividing cells in the body, particularly in the bone marrow, gastrointestinal tract, and central nervous system. Symptoms range from nausea and fatigue to severe organ damage and death, depending on the radiation dose. Immediate medical intervention is crucial for survival, including treatments to boost immune function and reduce radiation damage. The market is experiencing positive growth trends over the forecast period. Advancements in radiation oncology for effective cancer treatment using radiation therapy along with growing approval and adoption of novel therapeutics for acute radiation syndrome are expected to drive the market forward. Furthermore, the increasing investment by key players for development of innovative treatment options is also expected to support the market growth during this time.
Market Size in USD Bn
CAGR5.2%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 5.2% |
| Market Concentration | High |
| Major Players | Statera BioPharma, NeoImmuneTech, Cellerant Therapeutics, Partner Therapeutics, Pluristem Therapeutics and Among Others |
please let us know !
Acute Radiation Syndrome Market Trends
As global events have made people more aware of the dangers and health risks posed by nuclear emergencies and radiation exposure, advancements in medical countermeasures for treating acute radiation syndrome have become increasingly important. Any nuclear emergency, whether due to accidents, meltdowns, or attacks, could potentially expose large populations to dangerous levels of radiation. While efforts are constantly being made to enhance safety and security worldwide, the risks of such disasters can never be fully eliminated. As a result of high-profile events over the past few decades like Chernobyl and Fukushima nuclear plant meltdowns, as well as lingering anxieties over the possibility of nuclear terrorism, public awareness of radiation health threats has reached unprecedented levels globally. People are now more knowledgeable about acute radiation sickness and its potential symptoms like nausea, vomiting, diarrhea, skin burns and injury to internal organs from very high radiation exposures. The potential scale of casualties from a large-scale event means there is heightened focus on developing medical responses that can effectively treat radiation sickness across entire populations in any affected area. Pharmaceutical companies and researchers recognize this growing market opportunity and need to advance more targeted and efficacious treatment protocols, diagnostic tools and therapeutic drugs for radiation injury. This climate of concern over nuclear/radiation emergencies and the prospect of helping medical organizations prepare for potential mass casualty scenarios is fueling greater investment and R&D in this sector.
Market Driver - Advancements in Biological Therapies and Radiation Countermeasures Provide New Avenues for Treatment.
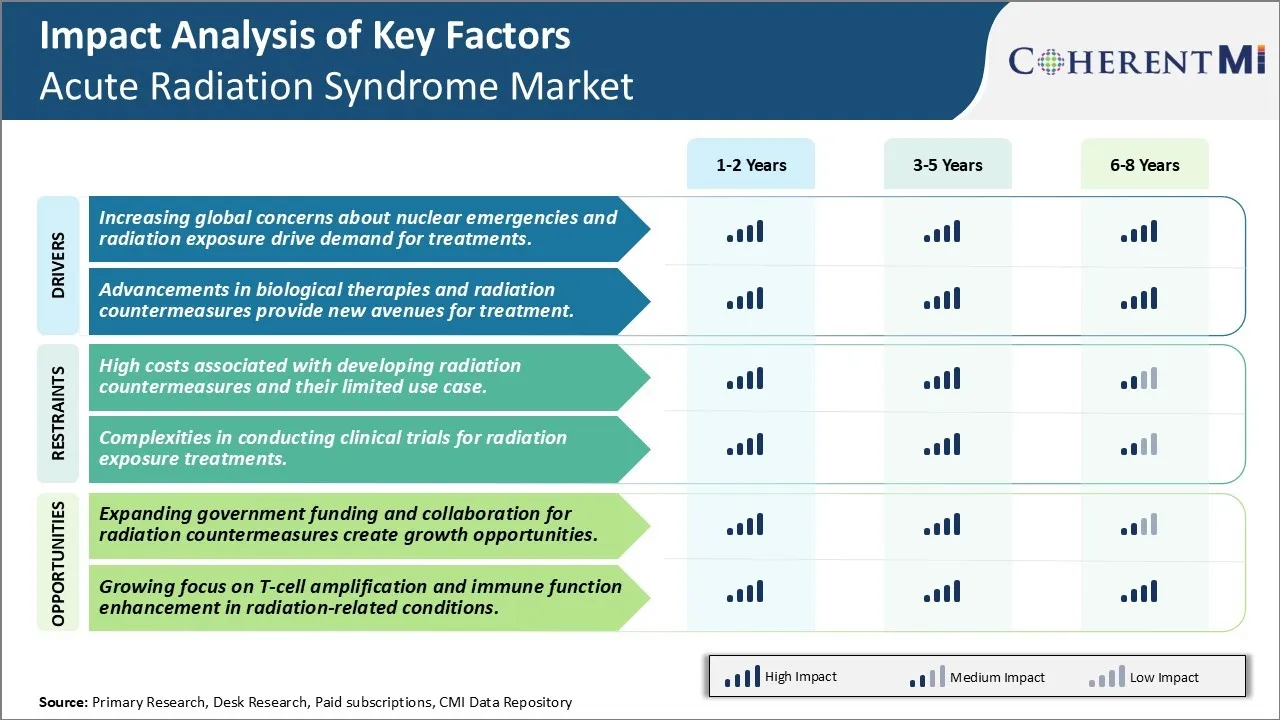
Market Challenge - High Costs Associated with Developing Radiation Countermeasures and Their Limited Use Case.
The acute radiation syndrome market is presented with several growth opportunities owing to the expanding government funding and collaboration for research in radiation countermeasures. Many governments and global institutions recognize the importance of developing effective medical responses for radiation emergencies. They are supporting research through increased public funding and partnerships. For instance, the United States government is heavily investing in the development of medical countermeasures through the Biomedical Advanced Research and Development Authority. Similarly, the European Commission is funding projects that aim to improve medical preparedness. This stable funding pipeline reduces investment risks for companies. Additionally, cross-industry collaborations and consortium models are coming up with the aim of pooling resources as well as spreading costs and risks associated with research. Such initiatives have spurred several new product pipeline programs. They are also attracting participation from more players. The collective efforts are expected to speed up product development and commercialization, thereby driving the market forward.
Prescribers preferences of Acute Radiation Syndrome Market
Acute Radiation Syndrome (ARS) is typically treated in 3 stages - the prodromal, latent and recovery stages - depending on the severity of radiation exposure. In the prodromal stage with mild radiation exposure (<2Gy), prescribers generally opt for supportive care through hydration and antiemetics like ondansetron to manage nausea and vomiting.
In cases of severe radiation exposure (>6Gy), the recovery stage focuses on intensive CBC support along with broad-spectrum antibiotics to prevent sepsis. Prescribers commonly choose between Neupogen and Leukine (sargramostim) for hematopoietic growth factor support. Blood product transfusions are also widely used to replace lost cell lines.
Treatment Option Analysis of Acute Radiation Syndrome Market
Acute radiation syndrome (ARS) can be divided into four primary stages based on symptoms and prognosis - mild, moderate, severe, and acute. Treatment depends on the stage of ARS.
In severe ARS cases, bone marrow transplantation may be considered, especially if the entire bone marrow was exposed to high radiation doses. Transplantation replenishes the immune system. Common drugs used for transplantation include cyclophosphamide and total body irradiation as a conditioning regimen. Matching stem cells from a donor are then infused.
In summary, treatment depends on ARS staging, ranging from supportive care to growth factors to transplantation. Targeting the underlying bone marrow damage and restoring hematopoiesis are crucial given ARS impairs the body's ability to produce infection-fighting blood cells. Stem cell therapies show promise but transplants remain the standard treatment currently when feasible.
Key winning strategies adopted by key players of Acute Radiation Syndrome Market
Partnerships and Collaborations for Drug Development: In January 2024, BARDA (Biomedical Advanced Research and Development Authority) collaborated with Partner Therapeutics to develop and expand Leukine as a treatment solution for ARS. The partnership reinforces public health initiatives by ensuring better access to effective ARS treatments in emergency situations.
Focus on Research and Development of Advanced Treatment Options: In 2023, Pluristem Therapeutics designed PLX-R18 that promotes the regeneration of bone marrow and the recovery of blood cell production following radiation exposure.
Segmental Analysis of Acute Radiation Syndrome Market
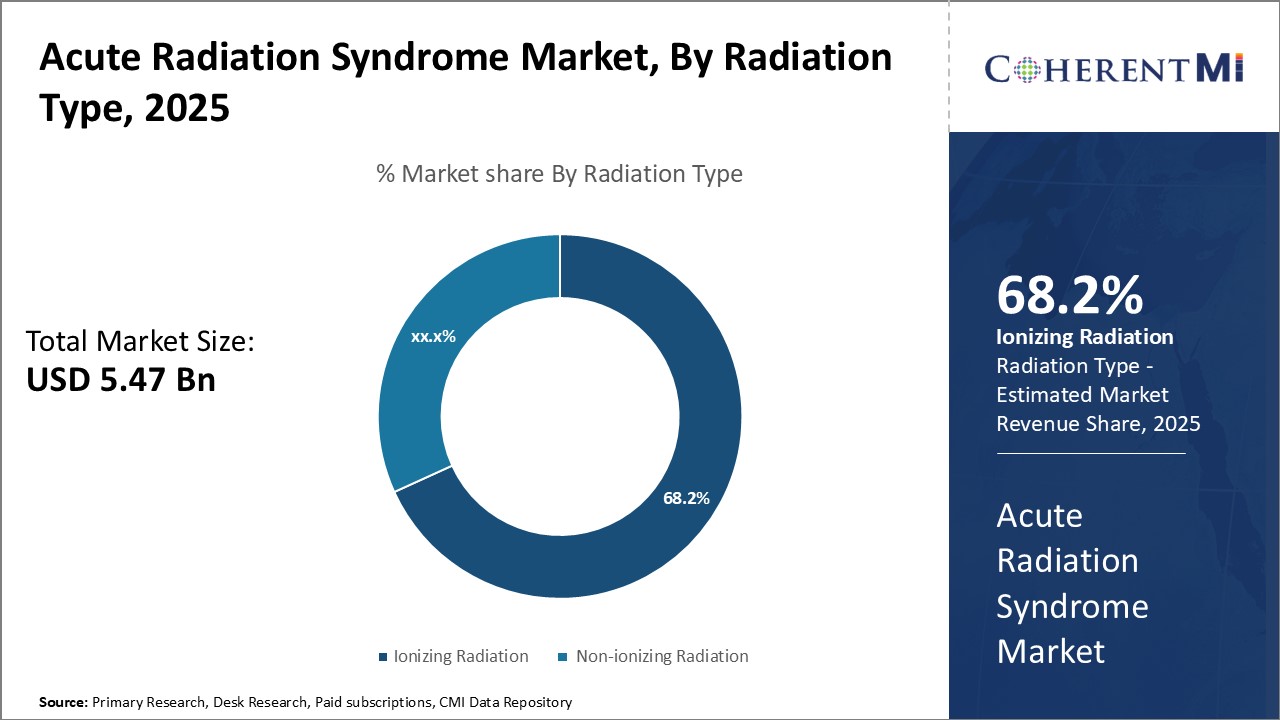
Insights, By Radiation Type, Rising Usage of X-rays and Radiation Therapy Drives Ionizing Radiation Segment.
The growing geriatric population prone to illnesses such as cancer has significantly increased the demand for diagnostic X-ray exams and radiation therapy sessions in recent years. Radiation therapy has become a standard treatment option either alone or in combination with surgery or chemotherapy for numerous cancer types. Advancements in radiation therapy techniques allowing higher precision and localized dose delivery have expanded its usage as well. Furthermore, awareness about the effectiveness of early cancer detection through diagnostic imaging is prompting more people to opt for routine full-body X-ray scans.
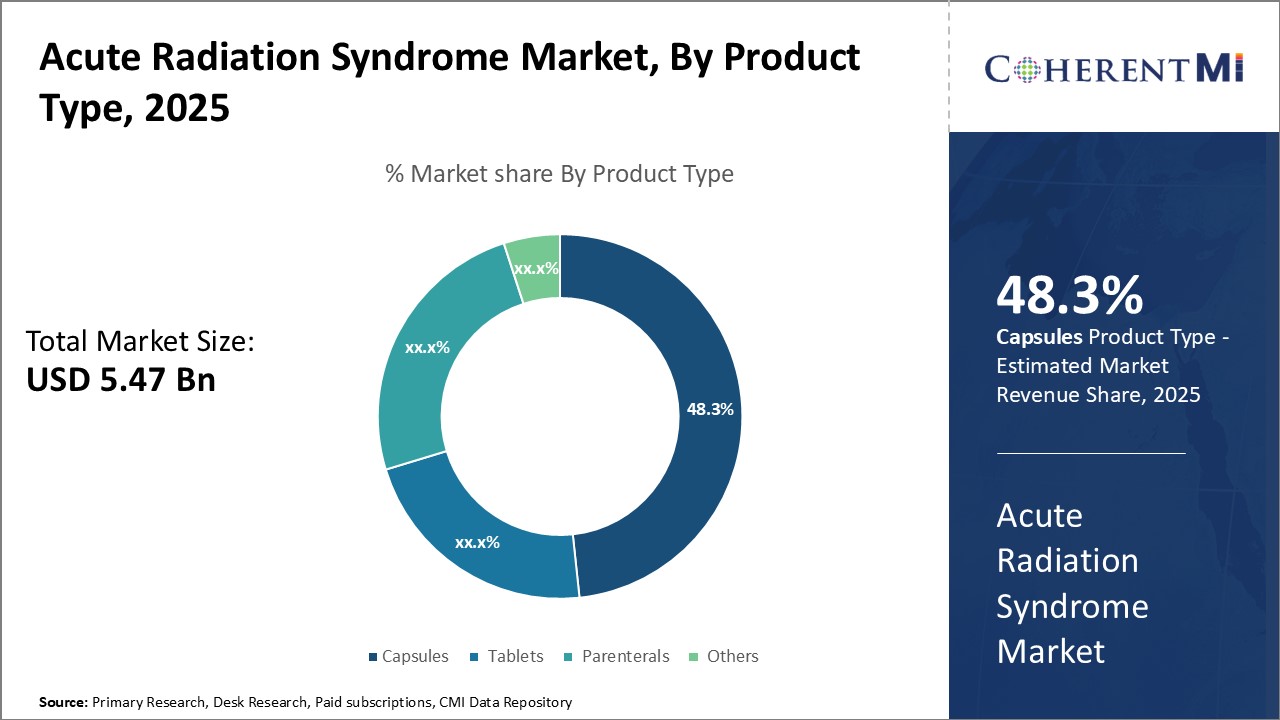 Insights, By Product Type, Popularity of Capsules Drives Highest Share of Acute Radiation Syndrome Treatment.
Insights, By Product Type, Popularity of Capsules Drives Highest Share of Acute Radiation Syndrome Treatment.Among various product types, capsules contribute the highest share 48.3% in 2025 owing to their popularity and widespread acceptance as the preferred oral dosage form. Capsules offer versatility in dosage and ease of administration compared to other oral options like tablets and liquids. They provide a number of formulation benefits that have cemented their top position in the market.
From a patient perspective, capsules are easy to identify based on their shape, color and imprints compared to uncoated tablets. They do not require water for ingestion and are less prone to choking risks in young, elderly or unconscious patients. Capsule shells offer an additional layer of security for accidental ingestion by young children compared to loose powders or even tablets. All these advantages over alternatives like parenteral injections have established capsules as the preferred delivery vehicle for oral acute radiation syndrome treatments.
Additional Insights of Acute Radiation Syndrome Market
Acute Radiation Syndrome (ARS) remains a significant public health concern, especially in the context of potential nuclear emergencies. The condition is marked by severe damage to organ systems, often leading to fatal outcomes without immediate intervention. STAT-600 by Statera BioPharma represents a major advancement in radiation countermeasures by activating toll-like receptors, which can enhance immune function and mitigate the effects of radiation. Similarly, NeoImmuneTech's NT-I7 is showing promise in preclinical studies by amplifying T-cells, offering a potential immune-modulatory approach to treating radiation syndrome. The ongoing development of these therapies highlights the urgent need for novel approaches to treating ARS, especially given the limitations of current treatments. With increased governmental support and collaboration between academic and private institutions, the ARS treatment landscape is poised for significant growth in the coming years. Advances in immunotherapy and targeted biological treatments may not only save lives but also improve the quality of care for individuals exposed to high doses of radiation.
Competitive overview of Acute Radiation Syndrome Market
The major players operating in the Acute Radiation Syndrome Market include Statera BioPharma, NeoImmuneTech, Cellerant Therapeutics, Partner Therapeutics, Pluristem Therapeutics, Humanetics Corporation, Amgen, Recipharm AB, Mission Pharmacal Company, Partner Therapeutics, Novartis AG and Mylan NV.
Acute Radiation Syndrome Market Leaders
- Statera BioPharma
- NeoImmuneTech
- Cellerant Therapeutics
- Partner Therapeutics
- Pluristem Therapeutics
Acute Radiation Syndrome Market - Competitive Rivalry

Acute Radiation Syndrome Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Acute Radiation Syndrome Market
- In May 2024, Statera BioPharma advanced its STAT-600 drug to Phase I trials, targeting immune enhancement as a countermeasure to radiation syndrome by stimulating toll-like receptors to mitigate radiation damage.
- In March 2024, NeoImmuneTech announced promising preclinical results for NT-I7, which showed potential in increasing T-cell amplification and restoring immune functionality after radiation exposure.
Acute Radiation Syndrome Market Segmentation
- By Radiation Type
- Ionizing Radiation
- Non-ionizing Radiation
- By Product Type
- Capsules
- Tablets
- Parenterals
- Others

Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Frequently Asked Questions :
How Big is the Acute Radiation Syndrome Market?
The Global Acute Radiation Syndrome Market is estimated to be valued at USD 5.47 bn in 2025 and is expected to reach USD 7.80 bn by 2032.
What will be the CAGR of the Acute Radiation Syndrome Market?
The CAGR of the Acute Radiation Syndrome Market is projected to be 5% from 2024-2031.
What are the major factors driving the Acute Radiation Syndrome Market growth?
The increasing global concerns about nuclear emergencies and radiation exposure drive demand for treatments and advancements in biological therapies and radiation countermeasures provide new avenues for treatment are the major factors driving the Acute Radiation Syndrome Market.
What are the key factors hampering the growth of the Acute Radiation Syndrome Market?
The high costs associated with developing radiation countermeasures and their limited use case and complexities in conducting clinical trials for radiation exposure treatments are the major factor hampering the growth of the Acute Radiation Syndrome Market.
Which is the leading Radiation Type in the Acute Radiation Syndrome Market?
Ionizing Radiation is the leading Radiation Type segment.
Which are the major players operating in the Acute Radiation Syndrome Market?
Statera BioPharma, NeoImmuneTech, Cellerant Therapeutics, Partner Therapeutics, Pluristem Therapeutics, Humanetics Corporation, Amgen, Recipharm AB, Mission Pharmacal Company, Partner Therapeutics, Novartis AG, Mylan NV are the major players.