Advanced Non-Squamous and Squamous NSCLC Market Trends
Market Driver - Higher Incidence of NSCLC due to Smoking and Environmental Risks
Lung cancer remains the foremost cause of cancer-associated mortality globally, and NSCLC accounts for approximately 80-85% of all lung cancer cases. Chronic exposure to cigarette smoke is the principal risk factor for the development of lung cancer. It is estimated that close to 90% of lung cancer deaths are caused due to tobacco smoking.
As per some recent epidemiological studies, the menace of air pollution has turned out to be an important environmental trigger for the development of lung cancers, especially in Asian countries where air quality is severely compromised. Areas with high levels of radon gas exposure in homes and workplaces have also witnessed elevated incidence of lung cancers. Workers in certain industries like mining, steel production, asbestos processing are at higher risk of lung cancer due to repeated inhalation of occupational lung carcinogens during their job tenure.
With continued urbanization and industrialization in emerging economies, exposure to outdoor and indoor air pollutants and toxic workplace atmospheres is likely to further propagate in the future, thereby fueling the rise of NSCLC cases.
Market Driver - Advancements in Targeted Therapies and Immunotherapy
Another prominent aspect bolstering the advanced non-squamous and squamous NSCLC market is the considerable progress made in the realm of targeted therapies and immunotherapy for lung malignancies. Traditionally, chemotherapy had been the mainstay of treatment for NSCLC patients.
However, over the past decade, there have been unprecedented advances in our understanding of cancer pathways and biological drivers of tumor growth. This has allowed researchers to identify novel molecular targets and develop matching targeted drugs that interfere with specific oncogenes or growth factor receptors critical for NSCLC cell proliferation and survival. Some examples include tyrosine kinase inhibitors blocking mutated forms of EGFR or ALK fusion proteins in selected patient subsets.
Additionally, immune checkpoint inhibitors unleashing the body's innate immune defenses have revolutionized lung cancer treatment. Drugs inhibiting PD-1/PD-L1 interaction and restoring anti-tumor immunity have shown durable responses and improved survival outcomes in advanced NSCLC, even after failure of chemotherapy and radiation. This has transformed NSCLC into one of the cancers most amenable to immunotherapy.
Wider availability and greater clinical use of these precision medicines and immunotherapies over the coming years will augment the advanced non-squamous and squamous NSCLC market size manifold.
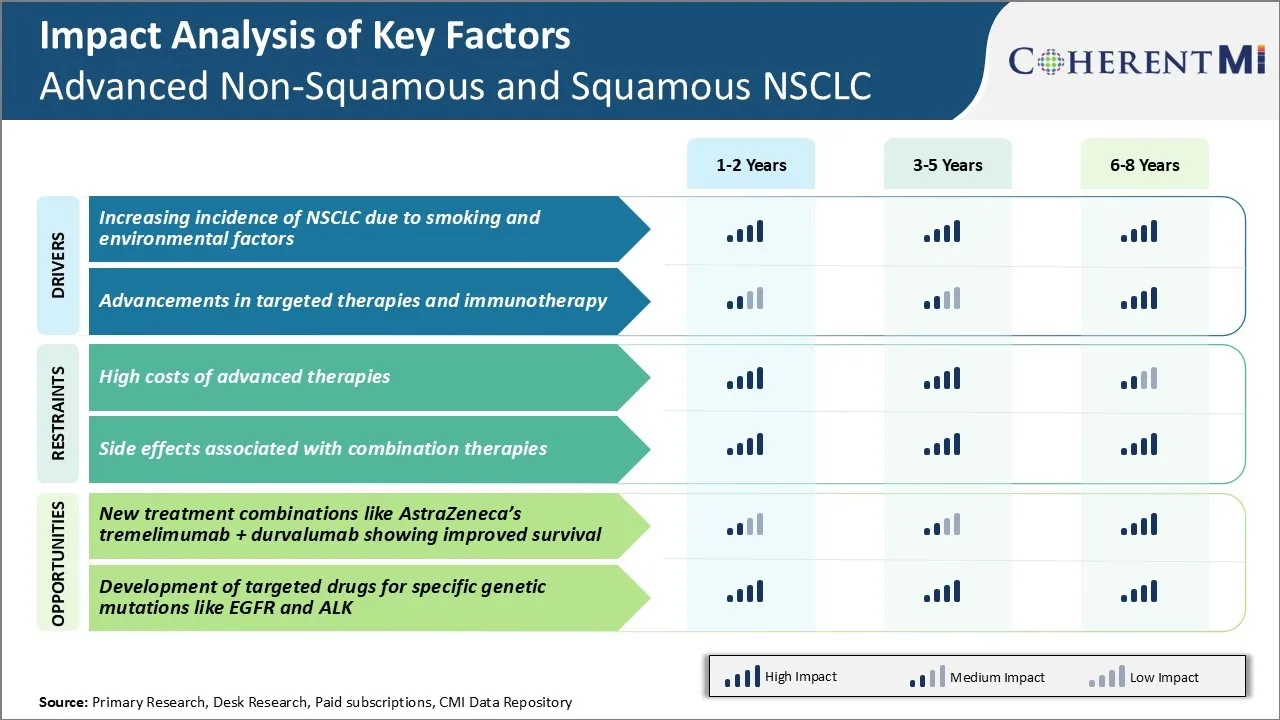
Market Challenge - High Costs of Advanced Therapies
One of the key challenges facing the advanced Non-Squamous and Squamous NSCLC market is the high costs associated with newer, advanced therapies. As immunotherapy and targeted therapies become standard of care options for frontline and later line treatment of NSCLC, the cost of these therapies poses a significant hurdle.
Most immunotherapy regimens cost over $100,000 annually, with some combinations even exceeding $200,000 for a year of treatment. While these advanced options have demonstrated improved survival outcomes compared to traditional chemotherapy, generating a better overall value proposition, the short-term costs remain exceedingly high. This price tag places significant financial pressure on healthcare systems and private payers.
With an aging global population and increasing incidence of NSCLC cases, the total cost of treating NSCLC patients threatens to swell healthcare budgets. Drug manufacturers must look at innovative pricing strategies and value-based agreements to make these life-saving therapies more affordable and accessible to a wider segment of patients.
Market Opportunity - New Treatment Combinations Showing Improved Survival
One major opportunity emerging in the advanced Non-Squamous and Squamous NSCLC market is the development of novel treatment combinations demonstrating improved survival. A prime example is AstraZeneca’s immunotherapy combination of tremelimumab and durvalumab.
Recent Phase 3 data from the MYSTIC trial showed that this dual checkpoint inhibitor regimen helped extend overall survival by close to 3 months compared to chemotherapy in frontline NSCLC treatment. At a median follow up of over 2 years, the combination delivered a 22% reduction in the risk of death. Such improvements in survival endpoints represent a turning point in NSCLC care.
As newer combinations like tremelimumab + durvalumab become integral first-line options, they have the potential to significantly prolong longevity while also enhancing quality of life for NSCLC patients. This success highlights immunotherapy combinations as a flourishing area that can drive sustained growth opportunities within the NSCLC therapeutics market.