Alpha Thalassemia Market Trends
Market Driver - Increasing awareness and research in genetic disorders such as Alpha Thalassemia, leading to earlier diagnosis and improved treatment approaches.
Over the past decade there has been a substantial increase in awareness initiatives undertaken globally to spread awareness about genetic blood disorders like Alpha Thalassemia. Several non-profit organizations and patient advocacy groups have been working diligently towards educating the general population as well as healthcare providers about the signs, symptoms, and available diagnosis and treatment options for such inherited conditions. Moreover, supported by government and private funding, extensive research activities are being carried out by academic institutions and pharma companies to gain deeper clinical insights into the pathophysiology and natural history of these rare diseases. This has significantly improved understanding about the disorder and helped in early detection.
Routine screening programs have been implemented in many countries to test pregnant women and newborns for common hemoglobinopathies. Prenatal screening and diagnosis options are also increasingly available to help expectant mothers assess risk and prepare for the challenges of caring for an affected child. Together, these efforts have led to more Alpha Thalassemia cases being diagnosed during fetal stage or early childhood itself compared to past when most patients would remain undiagnosed for years. Timely diagnosis allows for tailored care and interventions from beginning to alleviate symptoms and manage the condition properly. It also helps reduce complications and health impacts in the long run. All of these factors related to growing research and new insights are positively contributing to higher diagnosis rates and optimized care pathways for Alpha Thalassemia patients.
Market Driver - Promising Pipeline Therapies to Drive the Market
The Alpha Thalassemia treatment market is likely to see lucrative growth opportunities in the coming years driven by a swelling pipeline of novel drug candidates currently under development. Multiple pharmaceutical companies and biotech start-ups have recognized this as an area of unmet need and are actively working on potential disease-modifying therapies through diverse mechanisms of action. If successfully translated into approved drugs, these innovative treatment options have the capability to transform the existing paradigms of care, particularly for non-transfusion-dependent cases of Alpha Thalassemia.
Some of the most advanced pipeline therapies which are expected to drive significant revenues include Mitapivat by Agios, Etavopivat by Protagonist Therapeutics, and Luspatercept by Acceleron/Celgene. Mitapivat is a first-in-class, oral small molecule pyruvate kinase activator which has shown promising results in effectively treating the underlying red blood cell dysfunction in Phase II studies and is currently in Phase III trial. Likewise, Etavopivat, another oral pyruvate kinase activator, has demonstrated reduction in extravascular hemolysis in early trials. Luspatercept, a late-stage investigational erythroid maturation agent, has completed Phase III successfuly and could be the first FDA approved therapy specifically intended for non-transfusion-dependent Thalassemia patients.
If approved, these novel agents may offer patients an oral treatment option to significantly reduce transfusions and build healthier red blood cells without regular hospital visits for transfusion support. They will also help address an unmet need for treating the non-transfusion subset of patients who until now had minimal approved therapeutic options available. This pipeline progress is expected to herald a new age in Alpha Thalassemia management over the next five years and beyond.
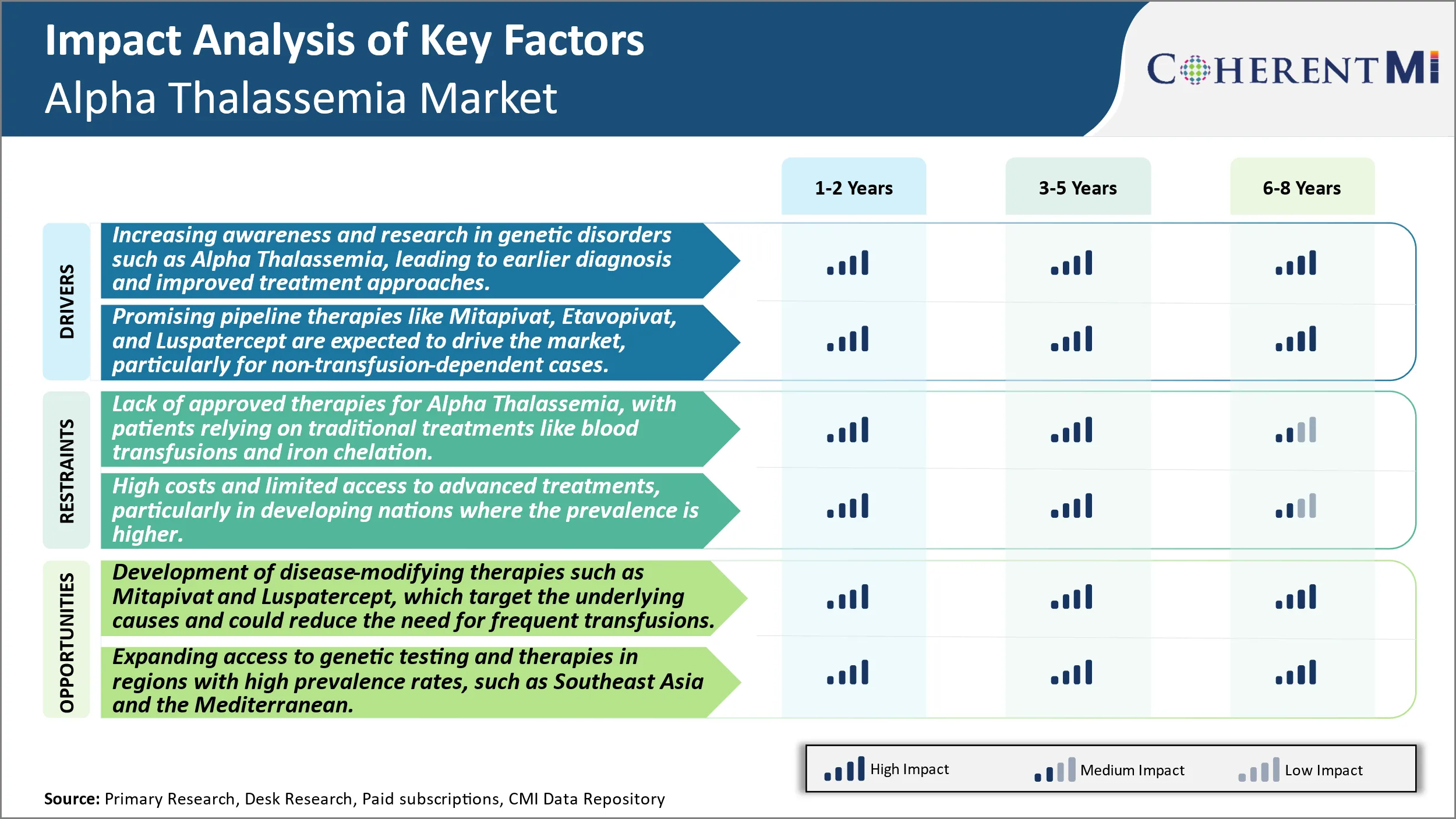
Market Challenge - Lack of Approved Therapies for Alpha Thalassemia, With Patients Relying on Traditional Treatments Like Blood Transfusions and Iron Chelation.
The lack of approved disease-modifying therapies for alpha thalassemia poses a significant challenge for both patients and the industry. Patients are currently reliant on supportive measures like regular blood transfusions and iron chelation to manage severe anemia symptoms. However, these traditional treatments are not a cure and require life-long adherence which can impact quality of life. Blood transfusions carry risks of iron overload in the long run if not managed properly with chelation. The needs of regularly monitoring iron levels and administering chelation Intravenously or orally places a treatment burden on patients. From an industry perspective, the lack of curative or disease-modifying treatment options limits growth potential. Pharmaceutical companies have fewer incentives to invest heavily in drug research and development for a rare disease with only supportive care options available. Overall, the unmet medical need of a cure drives both patient and industry interest in novel therapies that can target the underlying pathology.
Market Opportunity: Development of Disease-Modifying Therapies to Create Novel Opportunities.
There is promising opportunity in the developmesnt of disease-modifying therapies for alpha thalassemia that target the root cause of ineffective hemoglobin production. Two drugs, Mitapivat and Luspatercept, are in late-stage clinical trials investigating their potential to reduce transfusion dependency. Mitapivat activates pyruvate kinase which could restore red blood cell health and function. In clinical studies so far, it has demonstrated reductions in transfusion burden for certain patients. Luspatercept works by binding and inhibiting proteins responsible for ineffective erythropoiesis, thereby enabling normal red blood cell maturation. Its approval for beta-thalassemia has laid the foundation for studies in alpha thalassemia. If successful, these therapies may help improve clinical outcomes by enabling fewer transfusions and reducing iron overload management over the long term. Their approval could reinvigorate the market by fulfilling the critical need for curative or disease-modifying treatments for alpha thalassemia patients.