Antibody-mediated Rejection Market Trends
Market Driver - Increasing Number of Organ Transplants Globally
Growing awareness about organ donation, coupled with state-of-the-art surgical techniques, have enabled doctors to successfully carry out complex transplant procedures on a regular basis now. This has significantly boosted the total number of organ transplants performed worldwide each year. For instance, data from the Organ Procurement and Transplantation Network (OPTN) in the United States shows that the volumes have steadily increased over the past decade, with over 40,000 transplants done in 2021 alone.
However, despite the donor organ coming from a well-matched donor, there is always a risk of rejection by the recipient's immune system. Herein lies the role of medications that suppress the immune system or interfere with its rejection mechanism. With the rising transplantation rates globally, demand for anti-rejection drugs is growing in tandem.
Pharmaceutical companies are investing heavily in developing more effective and targeted immunosuppressants that can help improve transplant outcomes. Some newer agents even aim to induce immunotolerance, allowing transplant patients to eventually get off immunosuppression in the long run. All these factors point towards a continuously expanding patient pool who may potentially require lifelong anti-rejection medications, thus propelling the associated market forward.
Market Driver - Advancements in Therapies Targeting the Immune System's Rejection Mechanisms
Medical science continues advancing at a rapid pace, bringing numerous beneficial changes within the field of transplant immunology. Researchers have decoded intricate details about how the immune system recognizes non-self-tissues and initiates an attack. Pharmaceutical firms are leveraging immunological insights through dedicated R&D efforts focused on developing next-gen prophylactics.
Some examples include biological drugs inhibiting specific co-stimulatory molecules on immune cells or antibodies neutralizing inflammatory cytokines released during allograft rejection. Clinical studies evaluating these biologics show effective suppression of immune activation without broad suppression, translating to improved graft function and patient survival. Another promising arena is gene therapy whereby introduction or silencing of certain genes can help generate antigen-specific non-responsiveness or operational tolerance towards transplanted organs. Such targeted strategies are anticipated to replace conventional non-specific immunosuppressants over the long run.
Additionally, technologies like transplant tolerance induction through mixed chimerism hold promise. Here, bone marrow stem cells from the donor are transfused to establish mutual hematopoietic cell populations between donor and recipient. As outcomes from these novel therapies start yielding positive results in the coming years, they could transform anti-rejection medication use post-transplantation. This further validates strong growth dynamics for the overall market against this backdrop.
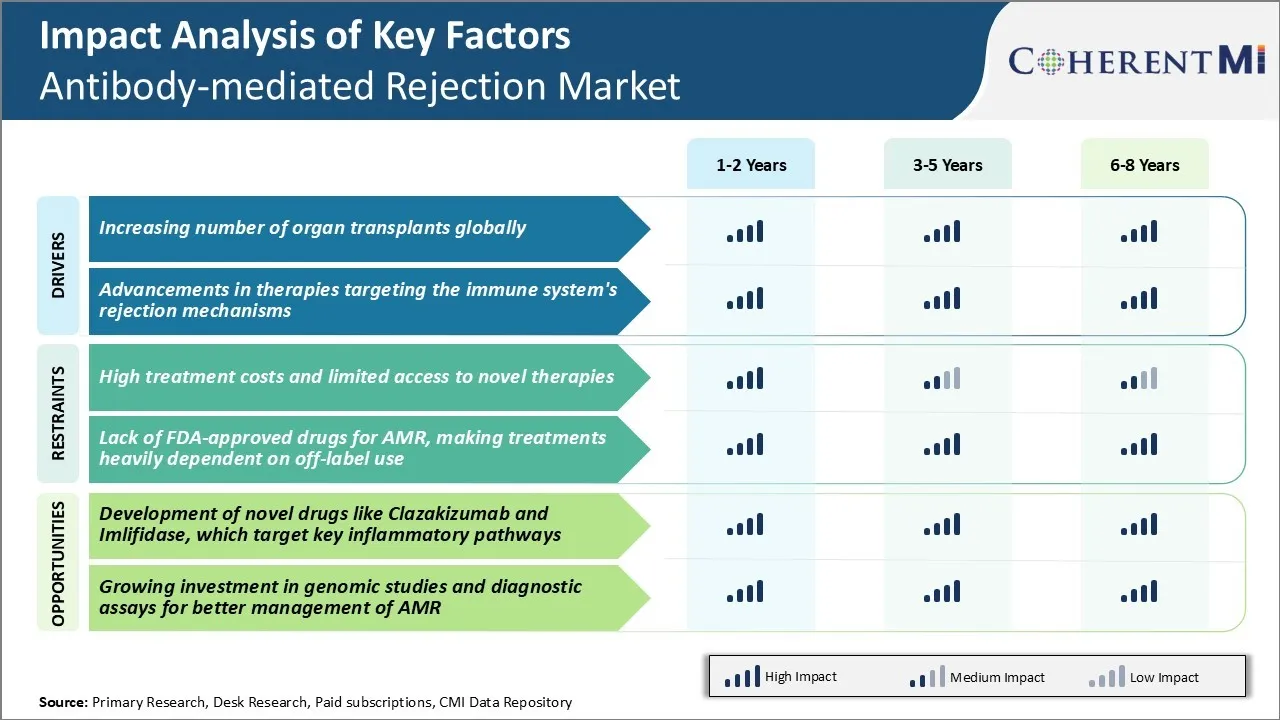
Market Challenge - High treatment costs and limited access to novel therapies
One of the major challenges currently faced by the antibody-mediated rejection market is the high costs of treatments and limited access to novel and emerging therapies. Antibody-mediated rejection treatments involve the use of immunosuppressive drugs such as infliximab, rituximab and intravenous immunoglobulin, which are very expensive for patients and healthcare systems to afford. The average annual drug cost for treating antibody-mediated rejection has been estimated to range from $20,000 to over $100,000 per patient. Additionally, many novel therapies that are still in development or have recently been approved are not widely accessible or affordable for all patients yet due to various factors. Partial coverage by insurance plans and health systems makes these newer treatment options out of reach for some patients. The high economic burden of antibody-mediated rejection treatments poses difficulties for broader patient access globally and threatens the sustainability of healthcare budgets. Wider adoption of novel and emerging therapies also depends on demonstrating superior clinical benefits over existing options as well as successful negotiations on drug pricing with payers. These access challenges must be addressed to improve treatment outcomes for more antibody-mediated rejection patients worldwide.
Market Opportunity - Development of Novel Drugs that Target Key Inflammatory Pathways
A major opportunity in the antibody-mediated rejection market lies in the development of novel drugs that target key inflammatory pathways associated with the condition. For instance, Clazakizumab is a monoclonal antibody against IL-6 that has shown promise in reducing acute rejection episodes in clinical trials. IL-6 is a major pro-inflammatory cytokine implicated in antibody-mediated injury to transplanted organs.
Similarly, Imlifidase is an enzyme therapy designed to enzymatically degrade IgGs which play a central role in triggering antibody-mediated rejection. In phase 2 trials, Imlifidase has demonstrated efficacy in desensitizing highly sensitized patients prior to kidney transplants.
As novel mechanisms of action, Clazakizumab and Imlifidase have potential to deliver superior clinical outcomes compared to existing therapies. Their approval in the coming years could help address unmet needs in patient subgroups with high antibody levels.
Furthermore, targeting new inflammatory pathways may yield options for synergistic combination therapies with immunomodulators. This represents a significant opportunity to improve patient prognoses and quality of life.