Cerebrotendinous Xanthomatosis Market Size - Analysis
The cerebrotendinous xanthomatosis market is estimated to be valued at USD 186.9 Mn in 2025 and is expected to reach USD 405.5 Mn by 2032, growing at a compound annual growth rate (CAGR) of 11.7% from 2025 to 2032. Recent discoveries about the genetic origins and treatment of the condition have driven increased diagnosis and treatment uptake over the past 5 years.
Market Size in USD Mn
CAGR11.7%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 11.7% |
| Market Concentration | High |
| Major Players | Alexion Pharmaceuticals, Inc., BioMarin Pharmaceutical Inc., Idorsia Pharmaceuticals Ltd., Intercept Pharmaceuticals, Inc., Merck & Co., Inc. and Among Others |
please let us know !
Cerebrotendinous Xanthomatosis Market Trends
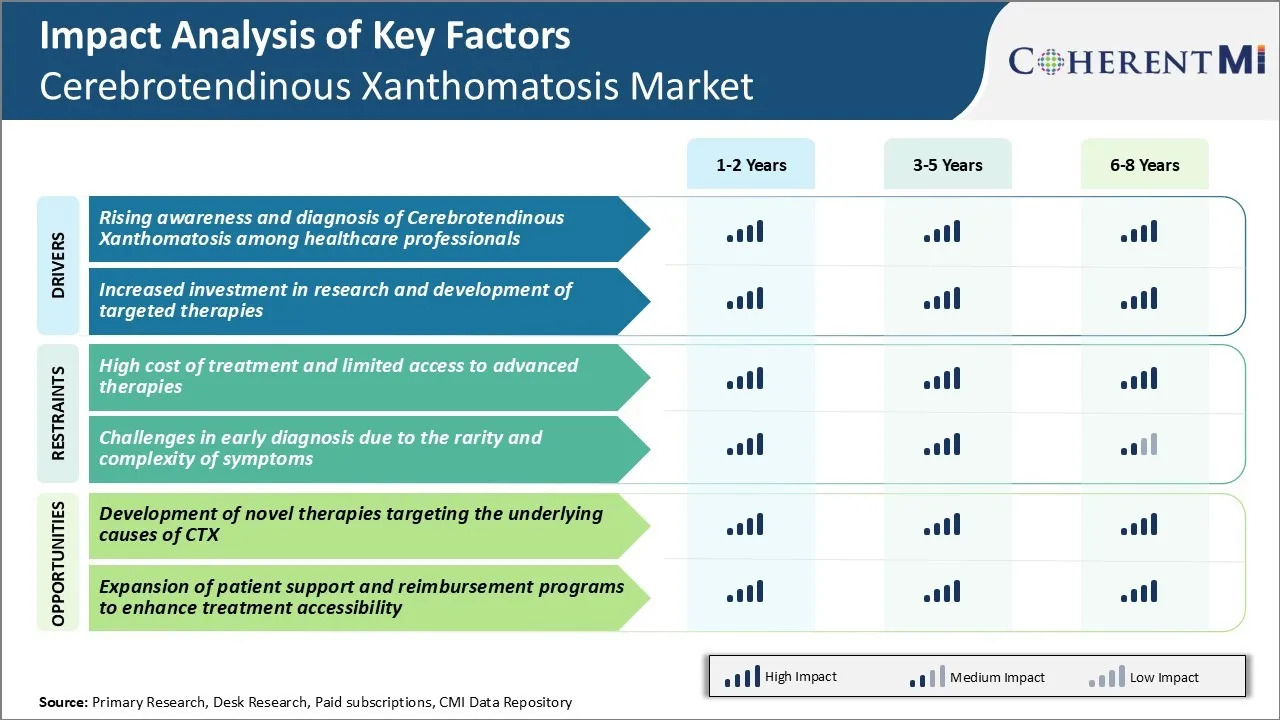
Market Driver - Rising Awareness and Diagnosis of Cerebrotendinous Xanthomatosis among Healthcare Professionals
With technological advancements in gene sequencing and diagnostic methods, healthcare professionals are now better equipped to diagnose even rare genetic conditions like cerebrotendinous xanthomatosis (CTX). Increased awareness about signs and symptoms of CTX through continued medical education and knowledge sharing platforms has helped clinicians to identify potential cases easily. This has significantly improved diagnosis rates globally in the last decade.
Now, even general physicians are capable of screening for the possibility of CTX after examining a patient's medical history and clinical manifestations. This allows for timely referral to genetic specialists and quick confirmation through molecular and enzymatic assays. Early diagnosis enables people to start cholesterol-lowering medication and make necessary lifestyle changes to curb disease progression.
In many countries, healthcare institutes and patient advocacy groups are striving to sensitize more medical practitioners about this rare genetic condition. Conferences, seminars and conference presentations dwell on topics like updated clinical management guidelines and promising research pipelines.
All these efforts combined have substantially increased the index of suspicion for CTX. More doctors are now able to recognize potential cases that may have been missed before. This certainly acts as a key driver for the growth of CTX market.
Market Driver - Increased Investment in Research and Development of Targeted Therapies
Rare diseases like CTX traditionally received less attention from pharmaceutical companies due to small affected patient pools and commercial viability challenges. However, the scenario is evolving gradually with growing investments in innovative research activities. Foundations and non-profit organizations are playing a pivotal role through fundraising drives and catalyzing partnerships between industry and academia. Crowdfunding campaigns are increasingly popular for financing orphan drug R&D.
A few dedicated biotechs have already made headway by developing first-in-class drugs that specifically target the underlying defects in CTX. With supportive clinical trial results, these newly approved medications have given new hope to patients. They substantially improve cholesterol metabolism and disease manifestations. An encouraging therapy pipeline continues to attract larger pharma players to co-develop combination therapies and delivery systems with the goal of achieving further improvement in treatment outcomes.
Backed by growing acceptance of high treatment prices for improving rare disease patients’ lives, venture capitalists are also not hesitating to fund cutting-edge projects. All these activities collectively provide strong momentum for novel therapy innovations. It works as a key growth driver by expanding treatment options and enhancing clinical management of patients over the coming years.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - High Cost of Treatment and Limited Access to Advanced Therapies
One of the major challenges currently faced in the cerebrotendinous xanthomatosis market is the high cost of treatment options available and limited access to advanced therapies for patients. CTX is a rare metabolic disorder and treatment options available are limited. The standard first line treatment involves a life-long therapy with chenodeoxycholic acid which needs to be administered orally on a daily basis.
However, chenodeoxycholic acid therapy is extremely expensive running into thousands of dollars annually for a single patient. This makes long term adherence to therapy a major issue due to the high costs involved. Additionally, not all patients respond adequately to this first line treatment and may require additional assisted therapies such as ursodeoxycholic acid or special dietary supplementation.
These alternate treatment options are even more expensive and not universally covered by insurance policies making them unaffordable for many patients. There is also a lack of availability of new disease modifying therapies in the CTX market. Currently, only symptomatic treatments are available and existing therapies target symptoms by controlling biochemical abnormalities and do not treat the root cause of the condition. This poses major challenges for people suffering from this rare genetic disorder.
Market Opportunity - Development of Novel Therapies Targeting the Underlying Causes of CTX
The cerebrotendinous xanthomatosis market presents significant opportunities for growth and innovation through development of novel targeted therapies. Currently, the condition is managed through symptomatic treatments available which lack effectiveness for all patients and are cost prohibitive for long term use. Therefore, there exists a high unmet need for development of new therapeutics that can potentially modify disease progression by targeting the underlying genetic or metabolic defects associated with CTX.
Promising areas for research include development of gene therapies using gene editing technologies like CRISP/Cas9 system to correct the genetic mutations responsible for the condition. Another opportunity lies in research towards small molecule therapies targeting key enzymes in the aberrant metabolic pathway leading to the pathological accumulation of toxic bile alcohols in CTX patients.
Development of effective and targeted disease modifying treatments can help manage the condition in a more effective and affordable manner if successfully developed. This presents substantial commercial opportunities for pharmaceutical companies to capitalize on in the coming years.
Prescribers preferences of Cerebrotendinous Xanthomatosis Market
Cerebrotendinous xanthomatosis is a rare lipid storage disorder characterized by tendon xanthomas and neurodegenerative manifestations. Prescribers follow a step-wise approach based on disease stage.
In early-stage disease with mild symptoms, lifestyle management focusing on a low-fat diet is the primary line of treatment. Prescribers may recommend over-the-counter supplements like fish oil to help control lipid levels.
As symptoms progress, medication is usually initiated. For moderate neurological involvement, prescribers typically start with chenodeoxycholic acid (Chenodol), an essential bile acid replacement. This helps reduce abnormal sterol levels and slows disease progression.
For advanced stage disease with severe neurological deficits, combination therapy is preferred. Prescribers commonly combine Chenodol with a cholesterol absorption inhibitor like ezetimibe (Zetia). This dual-action approach more effectively lowers cholesterol precursors that damage the brain and spinal cord over time.
For patients who show poor response, prescribers may switch to isotretinoin (Accutane), a retinoid drug. Though strong, it requires closer monitoring due to potential side effects on the liver, among other organs.
Overall treatment costs, insurance approvals, and degree of family support also impact medication choices. Prescribers endeavor to balance optimal clinical outcomes with maintaining a reasonable quality of life for patients.
Treatment Option Analysis of Cerebrotendinous Xanthomatosis Market
CTX is a rare disease with four main stages - early, intermediate, advanced neurological and end-stage. The primary treatment involves replacing the defective enzyme CYP27A1 using chenodeoxycholic acid (CDCA).
In early stage, when only tendon xanthomas are present, CDCA monotherapy at a dosage of 15 mg/kg per day is the preferred first-line treatment to prevent progression of the disease. CDCA helps replace the deficient enzyme and reduces cholesterol and bile acid levels.
In intermediate stage as neurological symptoms start, the treatment is augmented with a statin like atorvastatin along with CDCA. The combination helps lower low-density lipoprotein cholesterol and slow progression.
For advanced neurological stage with seizures or ataxia, the standard treatment remains CDCA along with atorvastatin. Additional medications like anti-seizure drugs or physical therapy may be used based on symptoms. Liver transplantation is also considered, as it can correct the enzymatic defect.
In end-stage with severe neurological impairment, the focus shifts to symptom management along with CDCA and statins. Living conditions are adjusted, and palliative care is instituted.
Overall, lifelong treatment with CDCA remains the mainstay. Addition of statins helps improve outcomes. Timely initiation and adherence to the prescribed regimen especially chenodeoxycholic acid can slow progression and avoid complications at later stages of CTX.
Key winning strategies adopted by key players of Cerebrotendinous Xanthomatosis Market
Product development and innovation: One of the major strategies adopted by key players such as Pfizer, Bristol-Myers Squibb, andothers has been continuousproductdevelopment and innovation. For example, in 2017, Pfizer launched a new medication called Zavesca to treat Cerebrotendinous Xanthomatosis (CTX). Zavesca (miglustat) was the first medication approved by the FDA specifically for CTX.
Targeted marketing and awareness campaigns: Companies like Bristol-Myers Squibb have launched targeted marketing campaigns focusing on rare disease communities and patient advocacy groups. They educate people about the symptoms and importance of early diagnosis of CTX. This helps create awareness and drives early screening. It also positions the company as a caring and patient-centric organization.
Partnerships and collaborations: Absence of competition has enabled companies to form strategic collaborations with hospital networks, research institutes, and genetic testing labs. For example, in 2010, Pfizer partnered with the US National Institutes of Health and academic medical centers to establish Centers of Excellence to improve diagnosis and care of CTX patients.
Pricing strategies: As CTX affects only a small percentage of the population globally, companies keep prices of drugs high to recover R&D investments. However, they also offer patient assistance programs and copay discount cards to improve affordability.
Segmental Analysis of Cerebrotendinous Xanthomatosis Market
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
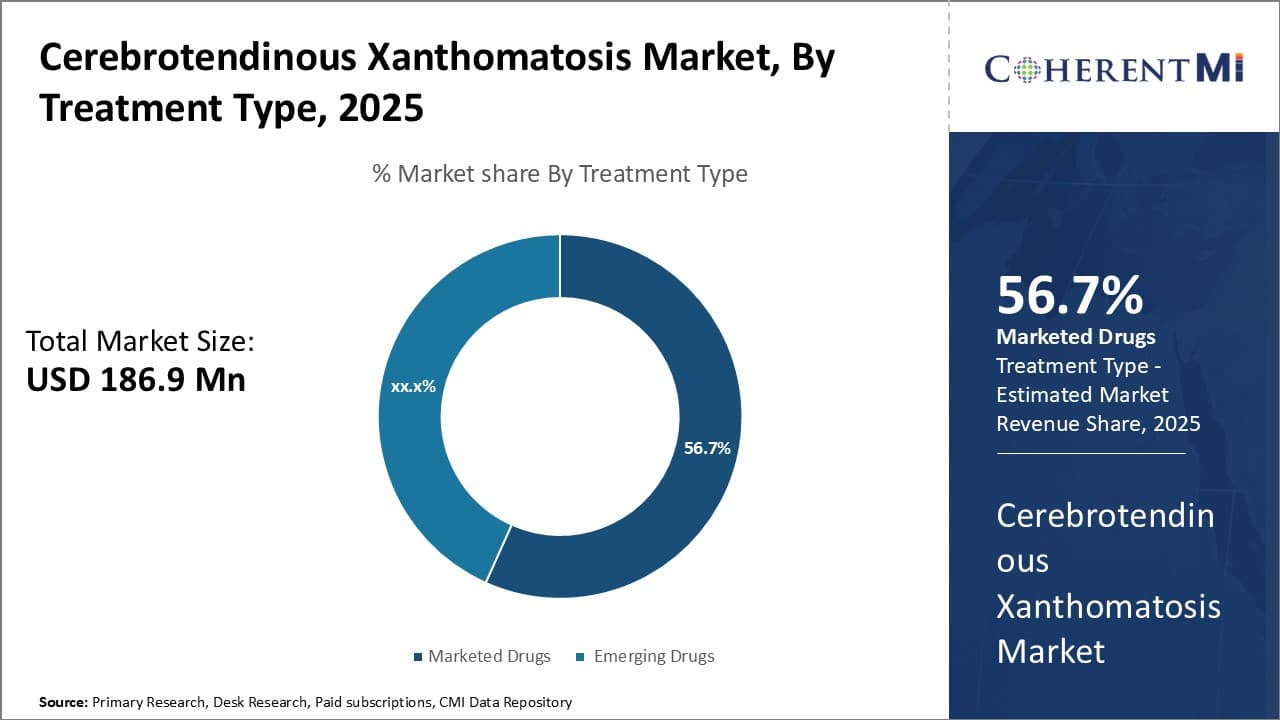
Insights, By Treatment Type: Marketed Drugs Grab Highest Market Share with Established Efficacy and Safety
The marketed drugs segment dominates the cerebrotendinous xanthomatosis market, which is estimated to account for 56.7% of the market share in 2025, due to the proven efficacy and safety profile of existing treatment options. Drugs such as chenodeoxycholic acid (CDCA) have long been the standard first-line treatment for Cerebrotendinous Xanthomatosis. CDCA effectively reduces abnormal lipid levels in the body by promoting biliary excretion of cholesterol and other sterols. Clinicians are highly familiar with CDCA's pharmacological properties and decades of clinical use have demonstrated its favorable benefit-risk balance in Cerebrotendinous Xanthomatosis patients. The consistency and predictability offered by CDCA compared to emerging drugs further entrenches its position as the therapy of choice for initial treatment.
Additionally, CDCA is generally well-tolerated with minimal side effects observed in the majority of patients treated over many years. Its strong safety record reduces risks faced by patients and gives peace of mind to physicians prescribing long-term therapy. This helps improve compliance with the treatment regimen relative to newer drugs. The established safety, efficacy and compliance advantages of existing drugs like CDCA drive sustained high usage and prescriber confidence, underpinning the marketed drugs segment's dominant share of the Cerebrotendinous Xanthomatosis therapeutic area. Emerging drugs aim to eventually capture a portion of this entrenched segment through superior profiles.
Additional Insights of Cerebrotendinous Xanthomatosis Market
- The CTX market is still under development, with a focus on finding therapies that can slow disease progression and potentially offer a cure. Government involvement and collaborations are crucial in funding research and making treatments available.
Competitive overview of Cerebrotendinous Xanthomatosis Market
The major players operating in the Cerebrotendinous Xanthomatosis Market include Alexion Pharmaceuticals, Inc., BioMarin Pharmaceutical Inc., Idorsia Pharmaceuticals Ltd., Intercept Pharmaceuticals, Inc., Merck & Co., Inc., Vivet Therapeutics, Corbus Pharmaceuticals, Nanobiotix, Biogen, Pfizer, Merck, and CG Oncology.
Cerebrotendinous Xanthomatosis Market Leaders
- Alexion Pharmaceuticals, Inc.
- BioMarin Pharmaceutical Inc.
- Idorsia Pharmaceuticals Ltd.
- Intercept Pharmaceuticals, Inc.
- Merck & Co., Inc.
Recent Developments in Cerebrotendinous Xanthomatosis Market
- Vivet Therapeutics received €4.9 million in funding from the French government in 2024 to advance the development of a gene therapy for cerebrotendinous xanthomatosis (CTX). This funding is part of the France Health Innovation Plan 2030 and aims to support the development of VTX-806, a gene therapy intended to stabilize or reverse symptoms of CTX. The funding will cover preclinical research, development activities, and manufacturing process development, as well as a clinical study to identify biomarkers for treatment effectiveness in CTX patients
- In 2023, Alexion Pharmaceuticals announced that it is advancing late-stage clinical trials for a novel CTX therapy aimed at addressing unmet needs in patient management.
- In 2022, BioMarin Pharmaceutical announced that it initiated a Phase III trial for an emerging CTX treatment that targets lipid metabolism pathways, promising improved patient outcomes.
Cerebrotendinous Xanthomatosis Market Segmentation
- By Treatment Type
- Marketed Drugs
- Emerging Drugs (Phase II, Phase III)
Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
