Chronic Fatigue Syndrome Market Size - Analysis
The chronic fatigue syndrome market is estimated to be valued at USD 13.86 Bn in 2025 and is expected to reach USD 20.03 Bn by 2032, growing at a compound annual growth rate (CAGR) of 5.4% from 2025 to 2032. Growing prevalence of chronic fatigue syndrome around the globe is the major factor driving the market's growth. Additionally, rising awareness regarding the availability of treatment is another important factor pushing the market upwards.
Market Size in USD Bn
CAGR5.4%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 5.4% |
| Market Concentration | High |
| Major Players | Hemispherx Biopharma, Inc., Cortene Inc., Mitsubishi Tanabe Pharma Corporation, NanoViricides, Inc., Tonix Pharmaceuticals Holding Corp. and Among Others |
please let us know !
Chronic Fatigue Syndrome Market Trends
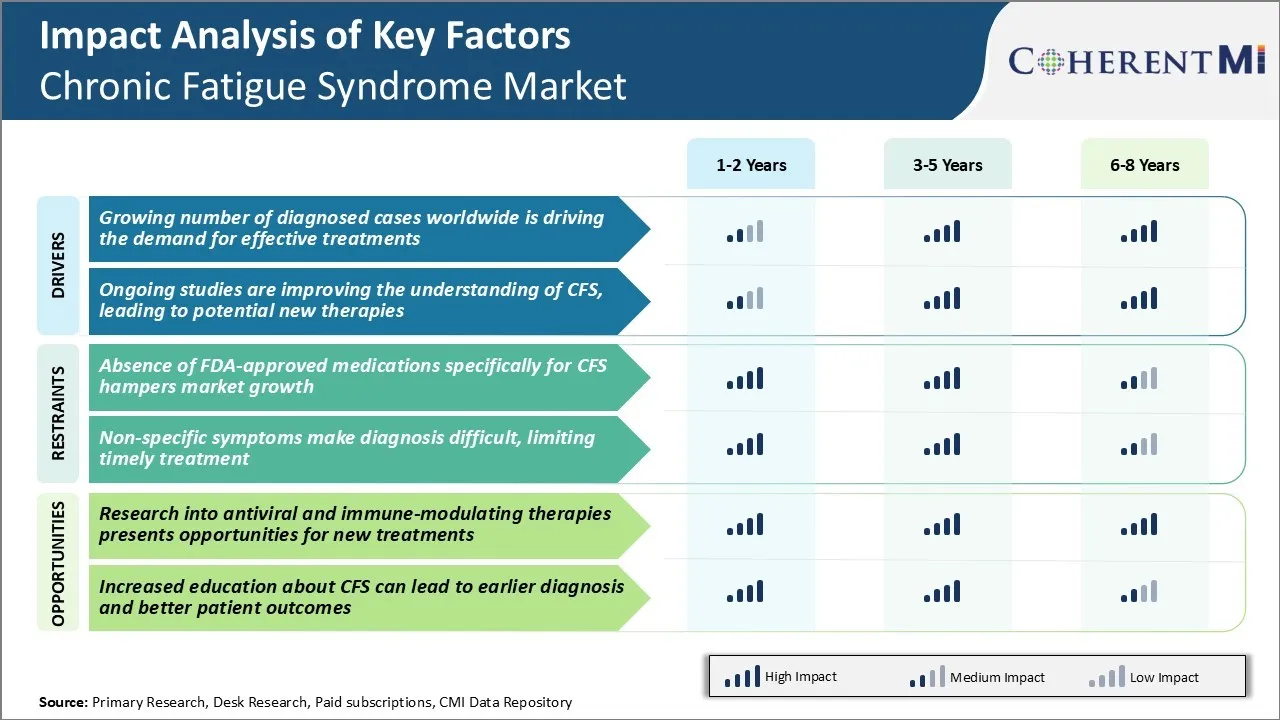
Market Driver - Growing Number of Diagnosed Cases Worldwide is Driving the Demand for Effective Treatments
The number of diagnosed cases of chronic fatigue syndrome (CFS), also known as myalgic encephalomyelitis, has been steadily increasing in the past few decades. As awareness about the condition has risen and diagnostic criteria have improved, more patients are being formally diagnosed with CFS by their physicians. A survey of primary care doctors and specialists in several countries found that over 20% of their patients presenting with prolonged fatigue ended up receiving a diagnosis of CFS after detailed medical evaluations ruled out other potential causes.
Many patients struggle with the severe fatigue, pain, cognitive difficulties and other symptoms for years without finding relief from conventional medications which are often ineffective for CFS. This has made them increasingly open to considering complementary and alternative therapies. Several observational studies have indicated benefits from graded exercise programs, cognitive behavioral therapy and alternative medicines like acupuncture for some cases.
Several clinical trials are now evaluating new medication candidates specifically designed for CFS. If proven safe and effective in large studies, these could transform management of the condition by finally offering patients much needed relief.
Market Driver - Ongoing Studies Improving Understanding of CFS
Research into chronic fatigue syndrome has seen considerable progress in recent times. Advanced investigative techniques are helping uncover disease mechanisms and signatures that provide deeper insights into the biochemistry of CFS. For example, brain imaging and microbiome analysis have revealed abnormal patterns of brain activity and gut microbial composition respectively in many patients as compared to healthy individuals.
Other studies have found signs of inflammation, oxidative stress, hormonal and immune system dysregulation in biological samples from CFS patients even when conventional medical tests appear normal. Such objective evidence of physiological disruptions offers strong validation that CFS is a real medical condition rather than just a psychological issue.
Investigators are also leveraging advanced tools like patient-derived stem cells and organoids grown in the lab to model the disease process and efficiently screen large libraries of drug candidates for those with the highest likelihood of success. If able to be replicated in larger cohorts, early encouraging signs from such targeted research efforts could soon translate into the first generation of rationally-designed treatments for CFS bringing long awaited relief.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - Absence of FDA-approved Medications Specifically for CFS Hampers Market Growth
The lack of FDA-approved drugs specifically indicated for CFS represents a major challenge for the growth of the chronic fatigue syndrome market. Currently, there are no medications that have received regulatory approval from the FDA for treating the core symptoms of CFS.
While some off-label drugs are used, their efficacy remains uncertain given the lack of large, high-quality clinical trials. This absence of approved pharmacological options limits treatment options available for doctors and frustrates patients who often experience debilitating levels of fatigue, pain, and neurocognitive difficulties.
Many patients experiment with numerous therapies in search of relief but frequently find solutions elusive. The dearth of approved medications also impacts the potential market valuation and attractiveness for pharmaceutical companies to make significant investments into research and development for CFS treatments. Until effective and approved drug therapies become available to help validate the pathology and legitimacy of CFS as a medical condition, growth in the market will likely remain constrained.
Market Opportunity - Research into Antiviral and Immune-modulating Therapies Presents Opportunities for New Treatments for Market
There is growing scientific evidence that dysfunction of the immune system and reactivation of latent viruses such as Epstein-Barr virus and human herpesvirus 6 may play a role in triggering or prolonging symptoms for some CFS patients. This has opened promising new areas for treatment research.
Several drug candidates that target immune system mechanisms or possess antiviral properties are currently in clinical trials. For example, antivirals that block viral replication or immune modulators that dampen inflammation hold hope as prospective treatment options. As studies further elucidate the pathophysiology of CFS, additional molecular pathways and targets are likely to emerge that could be modulated pharmaceutically.
A successful drug in these areas has the potential to capture a sizable portion of the CFS treatment market. Such a medication may also help establish biological legitimacy for the disease in medical and patient communities alike. This research momentum provides meaningful opportunities for pharmaceutical companies and investors over the coming years.
Prescribers preferences of Chronic Fatigue Syndrome Market
Chronic fatigue syndrome (CFS) is typically treated through a stepped approach based on the severity and symptoms at each stage of the disease. For mild cases at onset, doctors often recommend lifestyle modifications like exercise and stress management. Medications are generally not prescribed at this stage to avoid overmedicating and allow natural recovery.
As symptoms worsen at the moderate stage, prescription medications for specific issues commonly emerge. For pain symptoms, anticonvulsants like pregabalin (Lyrica) are frequently prescribed. For sleep disturbances, hypnotics such as zolpidem (Ambien) are given. For cognitive dysfunction, providers may trial stimulants including methylphenidate (Ritalin). If insomnia persists despite hypnotics, the off-label use of antidepressants fluoxetine (Prozac) or paroxetine (Paxil) may be considered.
For severe, debilitating cases that do not respond to initial treatments, prescribers tend to employ multidrug combination therapy targeting multiple symptom domains. Commonly used combinations include an antidepressant like duloxetine (Cymbalta) with an anticonvulsant such as gabapentin (Neurontin) to address pain, fatigue and mood issues. Provider experience with specific drugs, patient characteristics, insurance coverage, and side effect risks also influence medication choices at each stage of CFS. Overall adherence to clinical practice guidelines is important to optimize treatment outcomes.
Treatment Option Analysis of Chronic Fatigue Syndrome Market
Chronic fatigue syndrome (CFS) has distinct stages based on severity of symptoms. Mild CFS involves fatigue and minor impairment in daily activities. Treatment focuses on lifestyle management like exercise, diet, stress relief. Over-the-counter pain relievers and supplements may help.
Moderate CFS symptoms interfere significantly with functioning. First-line treatments are antidepressants like fluoxetine (Prozac) which help manage pain and sleep issues. Anti-viral medications target suspected viral causes and offer symptom relief. The combination of antidepressants and anti-virals is preferred due to multi-system pathology.
Severe CFS leaves patients largely home-bound or bed-ridden. The mainstay of treatment is graded exercise therapy (GET) involving rest periods and gradual increases in activity levels. This low-impact form of exercise aims to reverse deconditioning without overexertion. Cognitive behavioral therapy (CBT) helps patients adopt stress management techniques.
For relapse prevention, subsets of patients benefit from complementary treatments. Ampligen (poly I:poly C12U), an immunomodulator, reduces relapse when added to standardized medical care for its anti-inflammatory effects. Low-dose naltrexone modulates the immune system and pain pathways, improving overall function. For long-term management, these novel additions offer adjunctive benefits beyond first-line monotherapies.
Key winning strategies adopted by key players of Chronic Fatigue Syndrome Market
Focus on developing novel treatment options: One of the biggest unmet needs in the CFS market has been the lack of approved drug treatments. Recognizing this, major players have invested heavily in R&D to develop novel treatment options that target the underlying causes of CFS. For example, Hemispherx Biopharma began phase 3 trials in 2015 for their drug Ampligen, which targets viral immunomodulation.
Adopt a multi-disciplinary treatment approach: Many key players have adopted a treatment strategy that goes beyond medications to incorporate non-drug interventions like cognitive behavioral therapy (CBT) and graded exercise therapy (GET). This multi-pronged approach aims to both treat symptoms and support lifestyle management of the condition. The National Institutes of Health adopted such a strategy in their 2015 CFS research plan. This multi-disciplinary model has shown promise and represents a more holistic view of CFS care.
Expand into areas of co-morbid conditions: As research has found overlaps between CFS and other conditions like fibromyalgia, multiple sclerosis etc., companies have strategically expanded their focus beyond CFS. For example, Hemispherx also develops therapies for autoimmune and inflammatory disorders. This allows them to address a larger patient pool and gain new revenue streams. By recognizing shared pathways, players widen their relevance and commercial opportunities.
Segmental Analysis of Chronic Fatigue Syndrome Market
 To learn more about this report, Download Free Sample Copy
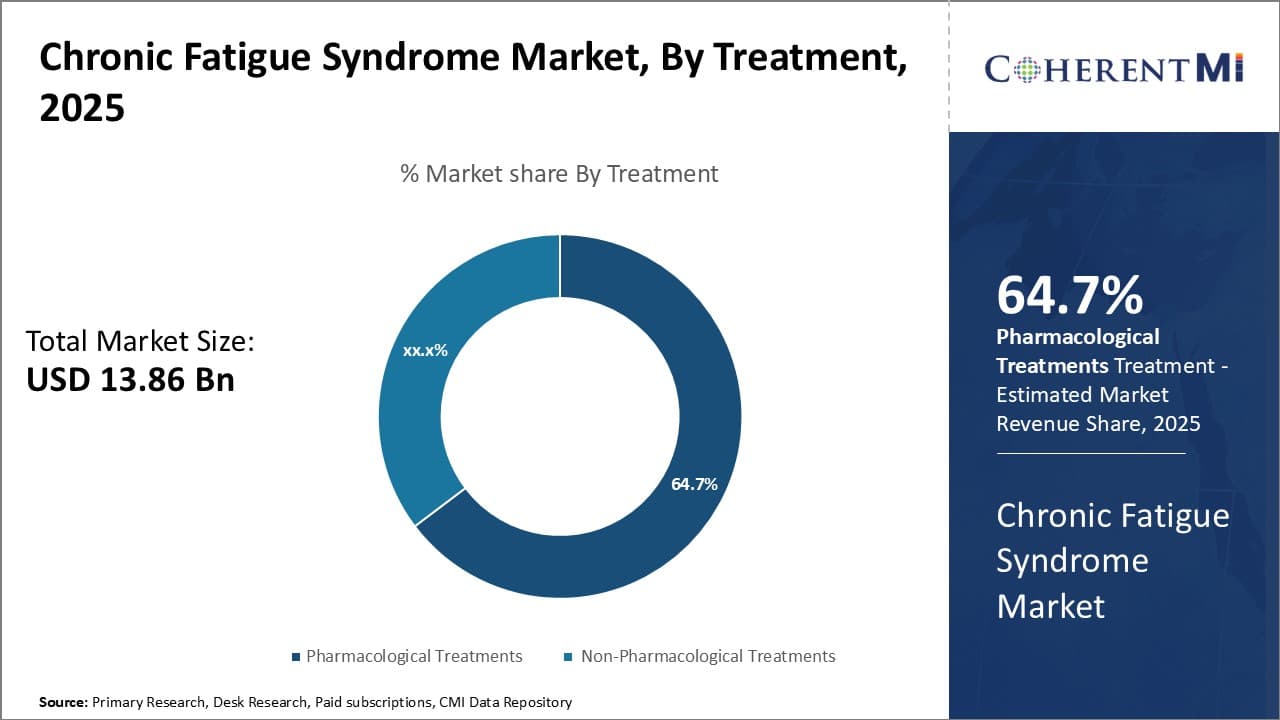
Insights, By Treatment: Preference for Effective Medication-Based Management Fuels Growth of Pharmacological Treatments Segment
To learn more about this report, Download Free Sample Copy
Insights, By Treatment: Preference for Effective Medication-Based Management Fuels Growth of Pharmacological Treatments Segment
In terms of treatment, pharmacological treatments are estimated to account for 64.7% share of the market in 2025, owning to its effectiveness in alleviating symptoms compared to other options. Many patients prefer treatment approaches backed by scientific evidence, and pharmacological interventions allow precise dosage and standardized composition to consistently deliver results.
Established prescription medications have extensive research supporting their safety and ability to target underlying causes via biological pathways. This provides assurance for those seeking a medically-validated solution. While non-drug remedies can complement pharmacology, the clinical rigor of medication validates it as many patients' first choice for control over fluctuating symptoms.
New drug development continues expanding treatment choice within this segment, aided by growing understanding of chronic fatigue syndrome's biological underpinnings.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
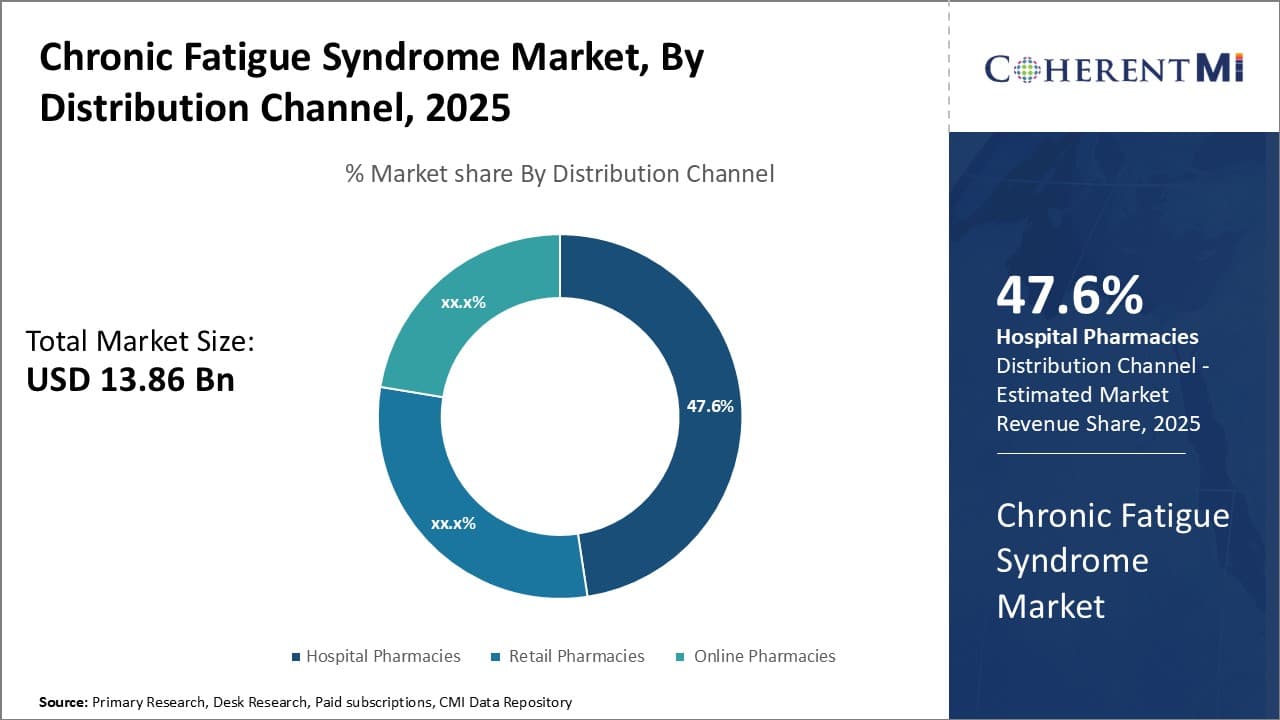
Insights, By Distribution Channel: Strategic Hospital Placement Maximizes Access to Specialized Diagnosis and Care
In terms of distribution channel, hospital pharmacies are projected to hold 47.6% of the market in 2025. This is because hospitals offer specialized chronic fatigue syndrome diagnosis and treatment centers, providing a centralized location for multidisciplinary care.
Being situated within hospitals allows these centers to leverage existing infrastructure and resources. It also integrates them into providers' clinical workflows, creating continuity as patients receive pharmacological therapy. Centralized care streamlines referrals between departments and enables comprehensive testing. Hospitals additionally employ fatigue specialists knowledgeable about the condition's complexities.
Their on-site availability supports accurately diagnosing different presentations and tailoring treatment plans. For patients daunted by navigating a fragmented system, hospitals present a convenient one-stop option to address all aspects of their condition.
Insights, By Diagnosis Method: Specialized Tools and Expert Evaluation Aid Definitive Diagnosis
In terms of diagnosis method, physical examination contributes the highest share of the market. Though blood tests and imaging play supporting roles, physical assessment remains fundamental to ruling out alternative conditions and accurately diagnosing chronic fatigue syndrome.
Skilled clinicians rely on thorough physical exams to identify symptoms like tender lymph nodes and pinpoint their onset, pattern, and severity fluctuations. Exams also detect other comorbidities complicating the diagnosis. Being performed by specialists knowledgeable in the condition's diverse manifestations helps pin down unclear presentations.
Compared to tests, physical exams involve direct evaluation and discussion with patients to gain a comprehensive understanding of their history and fully consider diagnostic criteria. With no single pathognomonic test for the syndrome, expert physicians integrate physical findings with ancillary test results and symptom profiles to make well-informed diagnoses. Their specialized evaluation tools aid definitive identification of chronic fatigue syndrome versus other fatigue-causing diseases.
Additional Insights of Chronic Fatigue Syndrome Market
- Global Impact: CFS affects an estimated 17 to 24 million people worldwide, with a significant impact on quality of life and productivity.
- Gender Disparity: Women are diagnosed with CFS at a rate four times higher than men, indicating a potential need for gender-specific research.
- Economic Burden: The annual economic impact of CFS in the United States is estimated to be over $24 billion due to healthcare costs and lost productivity.
- Diagnostic Delay: On average, patients experience a 5-year delay from symptom onset to diagnosis, underscoring the need for better awareness and diagnostic tools.
Competitive overview of Chronic Fatigue Syndrome Market
The major players operating in the Chronic Fatigue Syndrome Market include Hemispherx Biopharma, Inc., Cortene Inc., Mitsubishi Tanabe Pharma Corporation, NanoViricides, Inc., and Tonix Pharmaceuticals Holding Corp.
Chronic Fatigue Syndrome Market Leaders
- Hemispherx Biopharma, Inc.
- Cortene Inc.
- Mitsubishi Tanabe Pharma Corporation
- NanoViricides, Inc.
- Tonix Pharmaceuticals Holding Corp.
Recent Developments in Chronic Fatigue Syndrome Market
- In June 2023, Hemispherx Biopharma announced positive results from a Phase III trial of Ampligen, showing significant improvement in CFS symptoms, potentially leading to FDA approval. Hemispherx Biopharma (now AIM ImmunoTech) conducted a Phase III trial of Ampligen to treat chronic fatigue syndrome (CFS), where the drug met its primary endpoint. The trial showed significant improvement in exercise tolerance among patients, which is a crucial measure of physical performance in CFS. Specifically, patients receiving Ampligen improved their exercise treadmill performance by 19.4% compared to 5.1% in the placebo group.
- In March 2023, Cortene Inc. initiated a Phase II clinical trial for its drug CT38, targeting the neuroendocrine system to alleviate CFS symptoms, marking a novel approach in treatment. Cortene Inc. has been working on CT38, a novel drug targeting the neuroendocrine system, specifically the CRF2 receptor, to alleviate symptoms of chronic fatigue syndrome (CFS). The drug has gone through Phase I trials with healthy subjects and is intended to down-regulate the CRF2 receptor, which is thought to be involved in CFS.
- In September 2022, Tonix Pharmaceuticals received Fast Track designation from the FDA for TNX-102 SL, a sublingual tablet aimed at improving sleep quality in CFS patients.
Chronic Fatigue Syndrome Market Segmentation
- By Treatment
- Pharmacological Treatments
- Antiviral Agents
- Immune Modulators
- Antidepressants
- Sleep Aids
- Pain Relievers
- Non-Pharmacological Treatments
- Cognitive Behavioral Therapy (CBT)
- Graded Exercise Therapy (GET)
- Counseling
- Pharmacological Treatments
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- By Diagnosis Method
- Physical Examination
- Blood Tests
- Brain Imaging
- By End User
- Hospitals & Clinics
- Research Institutes
- Home Care Settings
Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
