Cytomegalovirus (CMV) Infection Therapeutic Market Trends
Market Driver - The Rising Number of Immunocompromised Patients, Such as Those Receiving Organ Transplants or Those Living With HIV/AIDS, is Driving Demand for More Effective Therapies and Preventive Vaccines for CMV.
The rising number of immunocompromised patients who are more susceptible to CMV infection is a key driver of demand for improved treatment options. Patients who receive organ transplants must take immunosuppressive drugs to prevent rejection of the transplanted organ by their immune system. However, this leaves them vulnerable to infections, including CMV. According to studies, CMV infection is common after kidney transplantation, with 50-80% of recipients experiencing CMV infection within the first year. For lung and heart transplant patients as well, CMV infection is a serious threat, with infection rates as high as 60%. Timely treatment of CMV in these patients is critical to prevent end-organ disease.
The growing population of HIV/AIDS patients worldwide is another risk group that is increasingly prone to CMV infection and disease. As antiretroviral treatment has improved survival rates of HIV patients, CMV has emerged as an important viral pathogen affecting this immunocompromised population. While antiretroviral treatment can control HIV and restore some immune function, patients still remain at risk for CMV disease. Reports estimate that 20-40% of HIV/AIDS patients experience CMV infection or end-organ disease involving the retina, gastrointestinal tract or lungs in their lifetimes. With over 36 million people living with HIV globally according to UNAIDS, the large prevalence of this virus group continues to fuel the need for more effective anti-CMV treatment and prophylactic options.
Market Driver - Technological Advancements Facilitating More Effective Vaccines Encourages Industry Developments.
Scientific and technological progress, especially in nucleic acid-based vaccine platforms, is opening up new possibilities for developing safe and effective anti-CMV vaccines. One example is Moderna's MRNA-1647 vaccine candidate, which utilizes a two-antigen MRNA approach to induce immunity against both the pentamer complex and envelope glycoprotein B (gB) of CMV. Pentamer complex is involved in CMV entry into host cells, while gB mediates fusion between the viral envelope and host cell membranes during infection. By targeting these two key players, mRNA-1647 aims to prevent CMV from gaining entry and establishing infection in host cells.
A key advantage of the mRNA platform is its ability to prompt in vivo production of target antigens in host cells, thereby inducing robust cellular and humoral immunity against these antigens. mRNA vaccines do not contain live attenuated or inactivated virus, making them potentially safer than traditional vaccine approaches. Moderna has also demonstrated the ability to develop MRNA vaccines for other viruses like Zika, influenza and HIV in animal models, supporting the translation of mRNA-1647 into clinical studies. If proven safe and effective in clinical trials, a single-dose mRNA-1647 could provide long-term protection against CMV and help curb its spread in high-risk groups. Technological innovations like these are expanding options for CMV prevention, a major driver of market growth.
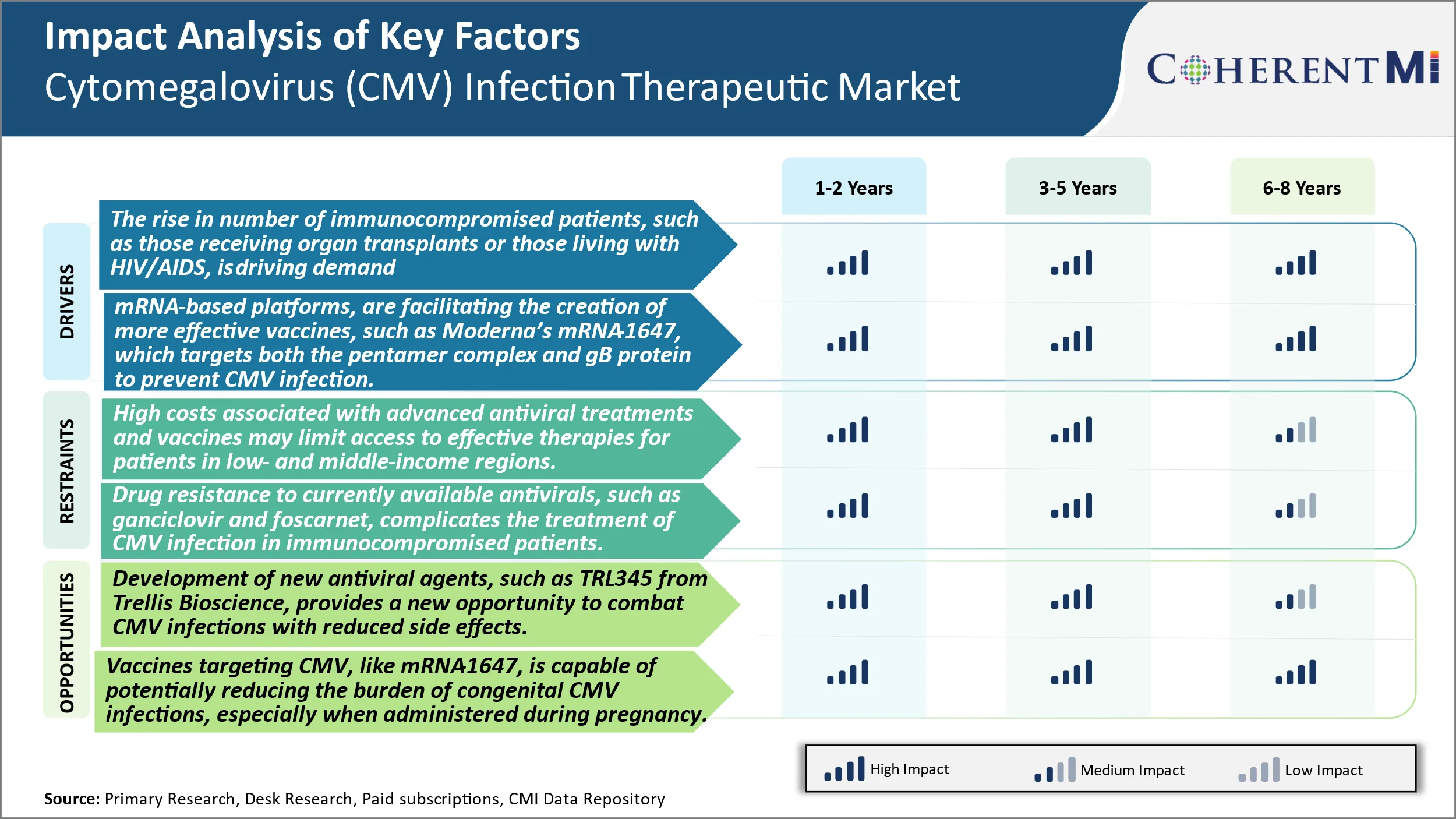
Market Challenge - High Costs Associated with Advanced Antiviral Treatments and Vaccines May Limit Access to Effective Therapies for Patients in Low- And Middle-Income Regions.
The development of advanced antiviral treatments and vaccines for cytomegalovirus (CMV) infections comes with considerable costs that can potentially restrict patient access in low- and middle-income regions. Developing new drug candidates and bringing them through the clinical trial process into regulatory approval involves enormous research and development investments by pharmaceutical companies. The pricing of new therapies targeting a niche market indication like CMV also needs to factor in the high costs to ensure reasonable returns. However, setting costs that are unaffordable for public health programs and individual patients in developing nations means a large section of the patient population remains underserved. Given the widespread distribution of CMV globally, this disparity in access to care poses a serious public health challenge. Innovative pathways and partnerships are needed to make new treatment options financially viable and geographically accessible.
Market Opportunity: Novel Candidates Offer Improved Options for Combating CMV.
The development of new antiviral agents targeting CMV provides an opportunity to address current limitations and better manage infections. Trellis Bioscience's TRL345 represents a promising candidate that acts on the conserved glycoprotein B site of the virus. This novel mechanism of action could enhance efficacy as resistance is less likely to emerge compared to existing therapies. Furthermore, focusing on a highly conserved region minimizes the risk of drug resistance developing due to viral mutations over time and geography. TRL345 has also exhibited a favorable side effect profile in early studies, raising hopes for improved tolerability. If successful in clinical trials, TRL345 may establish a new standard of care option able to combat a broader range of CMV strains. Its distinct profile could expand the commercial potential of the drug beyond current labels. This innovation illustrates how continuous R&D drives progress and enhances options to tackle challenging pathogens like CMV.