

The excessive daytime sleepiness (EDS) diagnosis market is estimated to be valued at USD 7.44 billion in 2025 and is expected to reach USD 13.95 billion by 2032, growing at a compound annual growth rate (CAGR) of 9.4% from 2025 to 2032.
The excessive daytime sleepiness (EDS) diagnosis market is growing at a steady rate due to the increasing awareness about sleep disorders and their diagnosis. Rise in lifestyle diseases globally is also leading to increased chances of developing sleep problems.
Market Size in USD Bn
CAGR9.4%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 9.4% |
| Market Concentration | Medium |
| Major Players | Jazz Pharmaceuticals, Takeda Pharmaceutical Company, Harmony Biosciences, Avadel Pharmaceuticals, Bioprojet Pharma and Among Others |
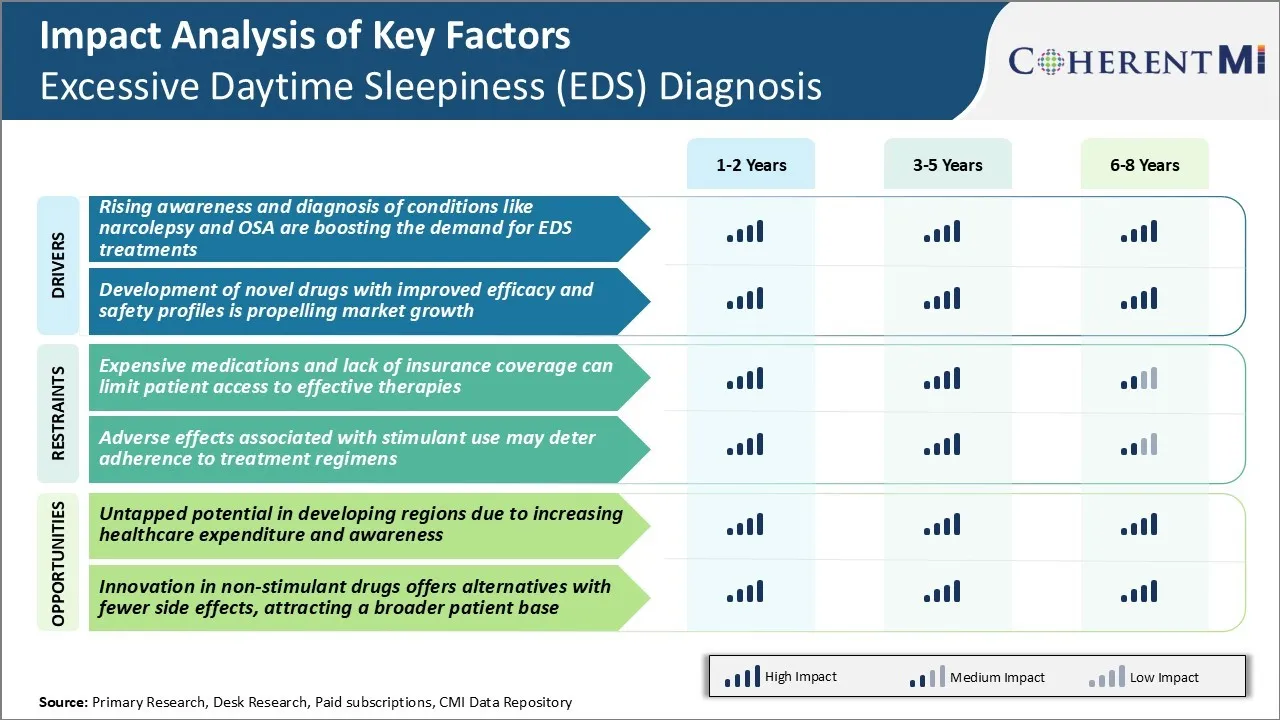
Market Driver - Rising Awareness and Diagnosis of Underlying Conditions like Narcolepsy and OSA
As researchers gradually gain better understanding about the underlying pathophysiology of excessive daytime sleepiness, they have been able to link several chronic sleep disorders to EDS symptoms. Prominent among them are narcolepsy and obstructive sleep apnea (OSA).
Research indicates that rising public knowledge about these chronic sleep conditions has encouraged more people to proactively seek medical advice for symptoms like daytime fatigue and sleepiness. Furthermore, advances in diagnostic techniques have allowed clinicians to more accurately diagnose conditions like narcolepsy and OSA in recent years. For example, multiple sleep latency tests (MSLT) play a crucial role in confirming narcolepsy diagnosis by objectively measuring a patient's average sleep latency. Similarly, overnight polysomnography or home sleep apnea tests are commonly employed to detect recurrent breathing obstructions during sleep episodes in suspected OSA patients.
As the number of diagnosed patients continue to grow, there is considerable demand for effective long-term treatments that provide relief from debilitating daytime sleepiness. Continuous positive airway pressure (CPAP) therapy remains the standard non-invasive treatment for moderate to severe OSA. The rising disease prevalence of underlying conditions directly influencing EDS presents a sizable opportunity for improved management options in the coming years.
Market Driver - Development of Novel Drugs
While currently available prescription therapies play a significant role in managing EDS symptoms, their long-term efficacy and safety continue to remain unideal for certain patients. This has driven extensive research within the pharmaceutical industry to identify new molecular entities with enhanced pharmacological profiles. Several promising drug candidates are in the clinical trial stages of development. For instance, novel wakefulness-promoting agents targeting orexin and histamine receptors show potential as an alternative to psychostimulants. Dual orexin receptor antagonists aim to balance sleep-wake cycles and consolidate sleep episodes.
On the other hand, gene therapies offer hope for long-lasting cures, especially in conditions with a clear genetic basis such as narcolepsy type 1. Researchers are also exploring cell-based therapies involving transplantation of orexin-producing cells. If proven to be well-tolerated and efficacious in humans, they could obviate the need for chronic medication use. Engineered formulations delivering already approved drugs in a controlled manner through novel routes also aim to improve adherence and outcomes.
The sizable R&D investments into developing more targeted therapeutics epitomizes the long-term growth prospects anticipated within the EDS treatment market. Successful approval and launch of novel drugs with improved efficacy, safety and amenability would significantly strengthen clinical management of EDS over current standard of care.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - Expensive Medications and Lack of Insurance Coverage Can Limit Patient Access to Effective Therapies
With the rise in awareness about EDS and its detrimental effects, the demand for effective diagnostic and treatment options has increased substantially. However, high costs of medications remain a major challenge impeding wider access and adoption.
Prescription drugs approved for EDS like Modafinil and Armodafinil, which are the mainstay of therapy, have a significantly high price tag ranging from hundreds to thousands of dollars per month. This financial burden falls largely on patients as out-of-pocket costs in most cases, given the lack of insurance coverage for such indications in many countries.
The stringent diagnostic criteria for conditions like Narcolepsy further limit the eligible patient pool qualifying for coverage or support. Additionally, certain developing regions have basic or no healthcare provisions for sleep disorders. With such economic barriers prevailing, only a small subset of patients who can afford it are actually able to receive recommended pharmacotherapy.
This ultimately results in significant unmet needs and inadequate management of EDS symptoms for much of the affected population globally.
Market Opportunity - Untapped Potential in Developing Regions Due to Increasing Healthcare Expenditure and Awareness
The EDS diagnosis market is poised to grow multifold presented with the immense opportunities unfolding in developing regions. These areas are witnessing substantial economic growth along with a concomitant rise in disposable incomes and healthcare spending in recent times. The growing affluence has bolstered the ability and willingness to pay for quality medical services.
With spreading medicalization and changing illness perceptions, sleep disorders are no longer considered a trivial concern. Improving awareness about sleep health and the repercussions of untreated EDS is driving more patients to actively seek appropriate diagnosis and treatment.
At the same time, these underpenetrated emerging markets have not been fully capitalized by global industry players due to lack of prior focus and infrastructure. However, they now offer a huge untapped reservoir with their large and steadily increasingly population bases. If unlocked strategically with need-based, cost-effective solutions and multi-stakeholder engagements, these regions can prove to be the next growth frontiers for businesses in the EDS diagnosis sector.
Excessive Daytime Sleepiness (EDS) is typically treated via a stepwise approach beginning with lifestyle modifications and progressing to pharmacological interventions if needed. For mild-moderate EDS, prescribers first recommend improving sleep hygiene and reducing sedentary behavior. However, if symptoms persist, prescribers may prescribe wakefulness-promoting medications.
For mild cases, the first-line treatment is typically modafinil (Provigil), a wakefulness-promoting agent that works in the hypothalamus. Modafinil is prescribed as a daytime treatment to boost wakefulness and alertness. For moderate-severe EDS, the first-line treatment is often sodium oxybate (Xyrem), a central nervous system depressant prescribed at bedtime to improve nighttime sleep quality and daytime wakefulness.
If patients do not respond to or tolerate first-line treatments, the next step involves switching or augmenting the treatment regimen. Prescribers may switch from modafinil to armodafinil (Nuvigil), its longer-acting enantiomer. Another option is adjunct RxA such as antidepressants, which are known to have wakefulness-promoting effects.
The severity of EDS symptoms, risk of side effects, insurance coverage, and patient preferences all factor into prescribers' medication selection. Ongoing patient follow-up is important to monitor response and make appropriate treatment adjustments over time.
EDS has various stages based on severity, from mild to moderate to severe. Treatment for EDS depends on determining the underlying cause and disease stage.
Mild EDS is often first treated with lifestyle modifications like maintaining a consistent sleep-wake schedule, exercising regularly, and avoiding naps. If lifestyle changes are insufficient, the first-line pharmacological treatment is usually a prescription stimulant. Modafinil (Provigil) is most commonly prescribed due to its effectiveness and mild side effect profile.
For moderate EDS, modafinil remains a standard first-line treatment option. However, some patients find it only partially effective or experience intolerable side effects. In such cases, the next treatment considered is often sunosi. Sunosi contains solriamfetol, a new dopaminergic and adrenergic receptor agonist. It offers an alternative for patients who do not respond adequately to or tolerate modafinil.
In severe EDS cases where monotherapy does not achieve sufficient results, combination therapy may be used. A common approach is adding a wakefulness-promoting agent like modafinil to treatment for the underlying cause, such as sodium oxybate for narcolepsy. For refractory narcolepsy specifically, which causes the most debilitating EDS, xywav is the treatment of choice. Xywav is a sodium oxybate formulation that allows split-night dosing for better symptom control throughout the day and night.
Product innovation - One of the most successful strategies adopted by leading players has been continuous innovation in diagnostic tools and techniques for EDS. For example, In 2018, Phillips launched SleepWorks Xcellent, a comprehensive sleep testing and diagnostic solution which combines ambulatory monitoring and in-lab polysomnography. This platform provides advanced sleep staging and visualization tools which help clinicians accurately diagnose sleep disorders like EDS. This innovative product helped Phillips gain a significant share in the sleep diagnostics segment.
Clinical trials and partnerships - Conducting collaborative clinical trials and partnering with research institutions and healthcare providers has helped companies gather real-world evidence to validate their diagnostic solutions. For example, in 2020, Itamar Medical collaborated with leading sleep clinics and hospitals to conduct EDS diagnostic accuracy trials for its WatchPAT device.
Focus on telehealth - With the growing demand for remote care during the pandemic, players have adopted telehealth-enabled strategies. In 2021, ResMed launched its Project Impel telehealth program in the UK which allows patients to undergo end-to-end sleep apnea diagnosis, including take-home monitoring and video consultations, remotely. This strategy addressed critical barriers and improved accessibility of EDS diagnosis globally. ResMed saw over 30% growth compared to pre-pandemic diagnostic volumes as a result.
 To learn more about this report, Download Free Sample Copy
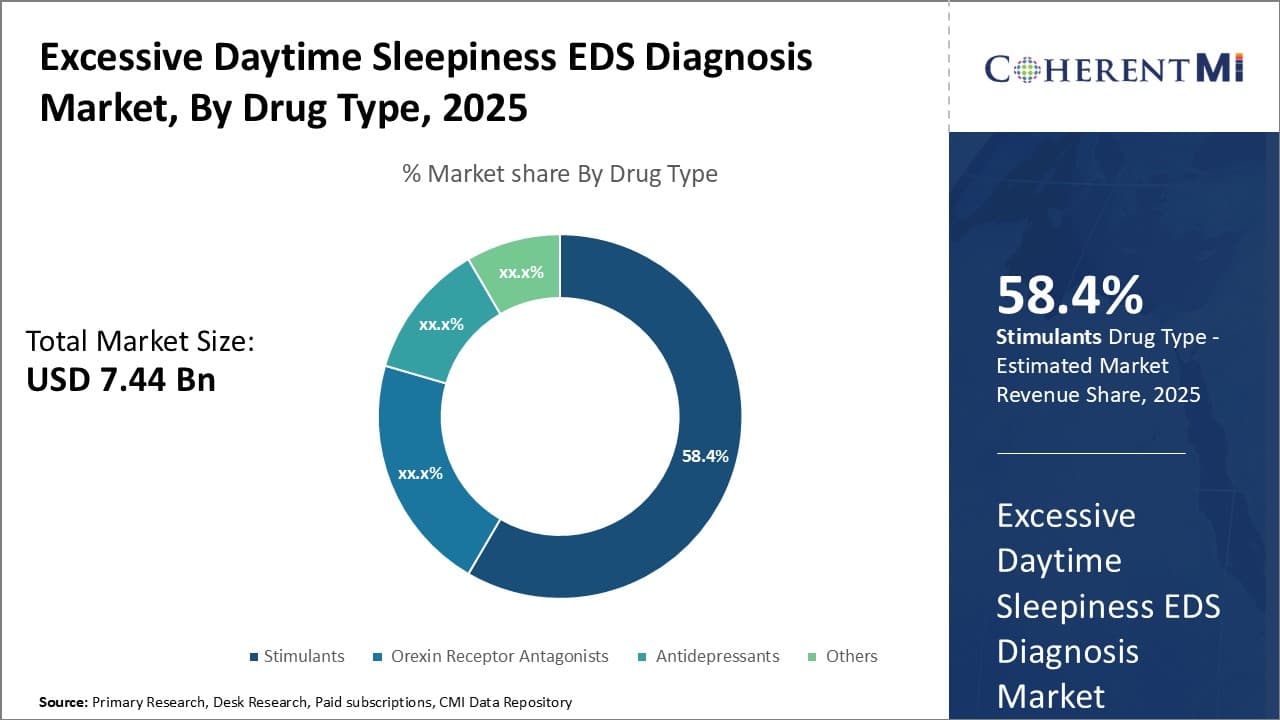
Insights, By Drug Type: Demand for Effective Wakefulness Promoting Agents Drives Growth Of Stimulants
To learn more about this report, Download Free Sample Copy
Insights, By Drug Type: Demand for Effective Wakefulness Promoting Agents Drives Growth Of Stimulants
In terms of drug type, stimulants contribute the highest share of the excessive daytime sleepiness (EDS) diagnosis market owning to the rising demand for effective wakefulness promoting agents. Stimulants work by increasing levels of dopamine and norepinephrine in the brain, chemicals that improve alertness and focus. Modafinil and armodafinil are the preferred stimulants owing to their favorable safety and tolerability profiles.
Additionally, stimulants tend to have a rapid onset of action along with longer durations of effect compared to other drug classes. This allows patients to achieve prompt symptom relief and better management of excessive daytime sleepiness.
Moreover, launch of newer long-acting stimulant formulations is further expanding the potential patient pool for stimulants. Ongoing research efforts to develop stimulants with enhanced pharmacokinetic properties will likely drive opportunities in this segment going forward.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
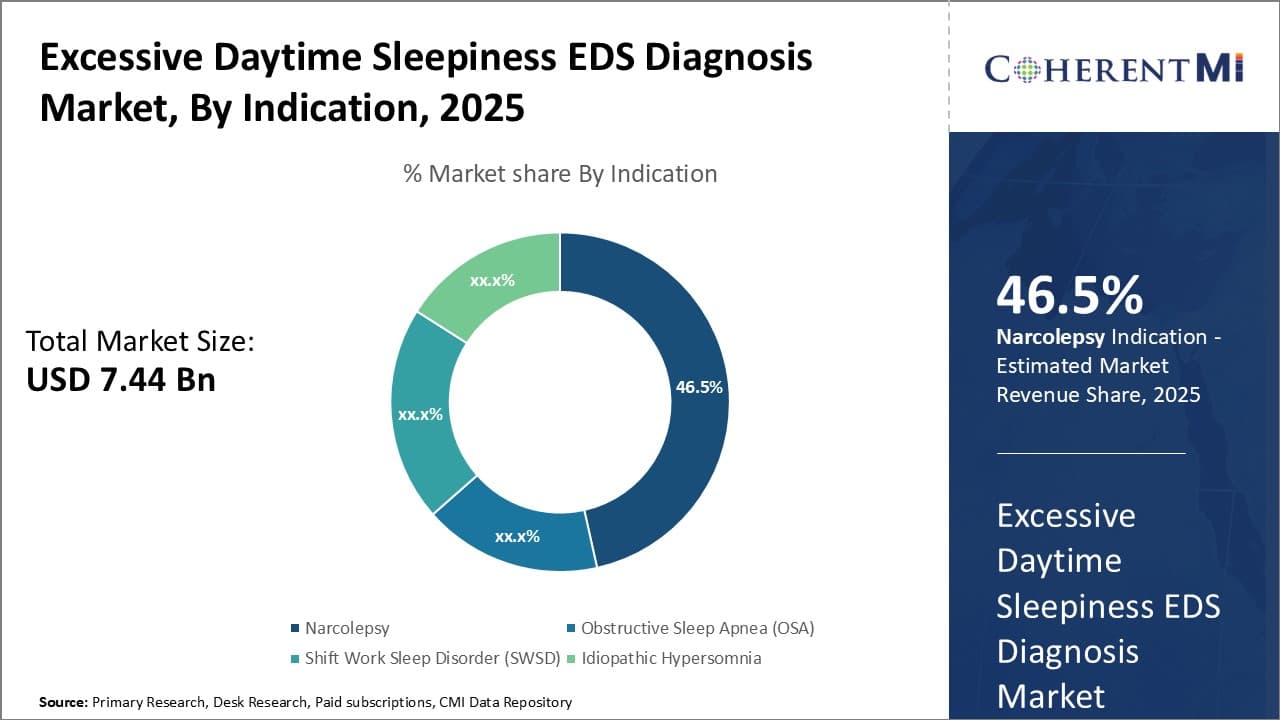
Insights, By Indication: Need for Improved Diagnosis and Management of Narcolepsy Fuels Growth
In terms of indication, narcolepsy contributes the highest share of the excessive daytime sleepiness (EDS) diagnosis market owing to the rising need for improved diagnosis and management of the disorder. Narcolepsy is characterized by excessive daytime sleepiness and episodes of daytime sleep attacks.
However, symptomatic diagnosis is often challenging given overlapping symptoms with other conditions. This has led to increased emphasis on biomarkers and polysomnography studies for definitive diagnosis of narcolepsy. Further, growing understanding of the underlying pathophysiology has resulted in availability of new targeted therapies.
Therefore, increasing focus on research and development of advanced diagnostics as well as new management approaches will likely aid the expansion of this patient segment.
Insights, By Distribution Channel: Convenience and Accessibility Increase Uptake Through Hospital Pharmacies
In terms of distribution channel, hospital pharmacies contribute the highest share owing to convenience and accessibility factors. Hospital pharmacies allow patients to procure wakefulness promoting medications easily during consultation visits or hospital admissions.
The availability of drugs through in-hospital dispensing desks circumvents the need for separate retail pharmacy visits. This proves significantly advantageous for narcolepsy patients experiencing recurrent daytime sleep attacks or cataplexy episodes.
Additionally, stored patient medical records facilitate continuous drug provision and management. The supervised care environment of hospitals also helps address concerns regarding medication adherence and safety. Collectively, these benefits serve to increase the proportion of sales occurring through hospital pharmacies for this therapeutic area.
The major players operating in the excessive daytime sleepiness (EDS) diagnosis market include Jazz Pharmaceuticals, Takeda Pharmaceutical Company, Harmony Biosciences, Avadel Pharmaceuticals, Bioprojet Pharma, Eisai Co., Ltd., Theranexus, Flamel Technologies, Orexigen Therapeutics, and Mitsubishi Tanabe Pharma Corporation.
Would you like to explore the option of buying individual sections of this report?
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Excessive Daytime Sleepiness (EDS) Diagnosis Market is segemented By Drug Type (Stimulants, Orexin R...
Excessive Daytime Sleepiness EDS Diagnosis Market
How big is the excessive daytime sleepiness (EDS) diagnosis market?
The excessive daytime sleepiness (EDS) diagnosis market is estimated to be valued at USD 7.44 billion in 2025 and is expected to reach USD 13.95 billion by 2032.
What are the key factors hampering the growth of the excessive daytime sleepiness (EDS) diagnosis market?
The expensive medications and lack of insurance coverage can limit patient access to effective therapies and adverse effects associated with stimulant use may deter adherence to treatment regimens are the major factor hampering the growth of the excessive daytime sleepiness (EDS) diagnosis market.
What are the major factors driving the excessive daytime sleepiness (EDS) diagnosis market growth?
The rising awareness and diagnosis of conditions like narcolepsy and OSA are boosting the demand for eds treatments and development of novel drugs with improved efficacy and safety profiles is propelling market growth are the major factor driving the excessive daytime sleepiness (EDS) diagnosis market.
Which is the leading drug type in the excessive daytime sleepiness (EDS) diagnosis market?
The leading drug type segment is stimulants.
Which are the major players operating in the excessive daytime sleepiness (EDS) diagnosis market?
Jazz Pharmaceuticals, Takeda Pharmaceutical Company, Harmony Biosciences, Avadel Pharmaceuticals, Bioprojet Pharma, Eisai Co., Ltd., Theranexus, Flamel Technologies, Orexigen Therapeutics, Mitsubishi Tanabe Pharma Corporation are the major players.
What will be the CAGR of the Excessive Daytime Sleepiness (EDS) Diagnosis Market?
The CAGR of the excessive daytime sleepiness (EDS) diagnosis market is projected to be 9.4% from 2025-2032.