Gene Therapy in Ophthalmology Market Trends
Market Driver - Rising Prevalence of Genetic Ocular Disorders Leading to Increased Demand for Effective Treatments
Genetic factors contribute significantly to the development of various ophthalmic conditions including retinitis pigmentosa, age-related macular degeneration (AMD), and glaucoma. Researchers have found that mutations in as many as 250 genes can result in retinal degeneration alone. The worldwide prevalence of such inherited retinal diseases (IRDs) is estimated to range from 1 in 2,000–3,000 persons.
Recent epidemiological studies further suggest that the burden of genetic eye disorders is rising continuously mainly due to increasing life expectancy globally. The growing patient pools facing debilitating vision loss due to genetic causes have generated a strong need for groundbreaking treatments. Conventional options such as medication and surgery lack efficacy in arresting disease progression driven by genetic defects.
Gene therapies, however, offer the promise of long-term vision restoration or preservation by precisely targeting the underlying genetic roots of ocular disorders. This potential for gene therapies to provide a curative outcome through a single administration has spurred significant interest from biopharmaceutical firms. It has also encouraged greater patient enrollment in ongoing clinical trials evaluating candidate therapies for conditions like Leber congenital amaurosis and AMD.
Market Driver - Advances in Gene Editing Technologies Enhancing the Development of Novel Therapies
Over the past decade, revolutionary technologies for gene manipulation have enabled enormous progress in gene therapy research and product development. Notable among these are CRISPR-Cas9, zinc finger nucleases, and TALE nucleases which allow swift, accurate editing of DNA sequences. CRISPR in particular has revolutionized the field through its simplicity, versatility and cost-effectiveness. It has accelerated discovery and preclinical research by facilitating large-scale screening and targeted mutagenesis investigations.
Building on these platforms, scientists are gaining deeper insights into disease-causing gene mutations and the complex molecular mechanisms underlying retinal degeneration. This has led to the creation of several proof-of-concept animal models that emulate human genetic eye diseases. Such models have been instrumental in demonstrating proof-of-principle for novel therapeutic strategies like knocking-out defective genes, inserting functional gene copies and gene supplementation through viral vector delivery.
In tandem, the availability of advanced characterization tools is facilitating the selection of therapeutic candidates with ideal dosage, duration and tissue targeting attributes. Collectively, the accelerating pace of technological evolution is empowering scientists to translate ambitious gene editing concepts into promising therapeutics with attributes like one-time administration and life-long efficacy against previously untreatable genetic eye conditions.
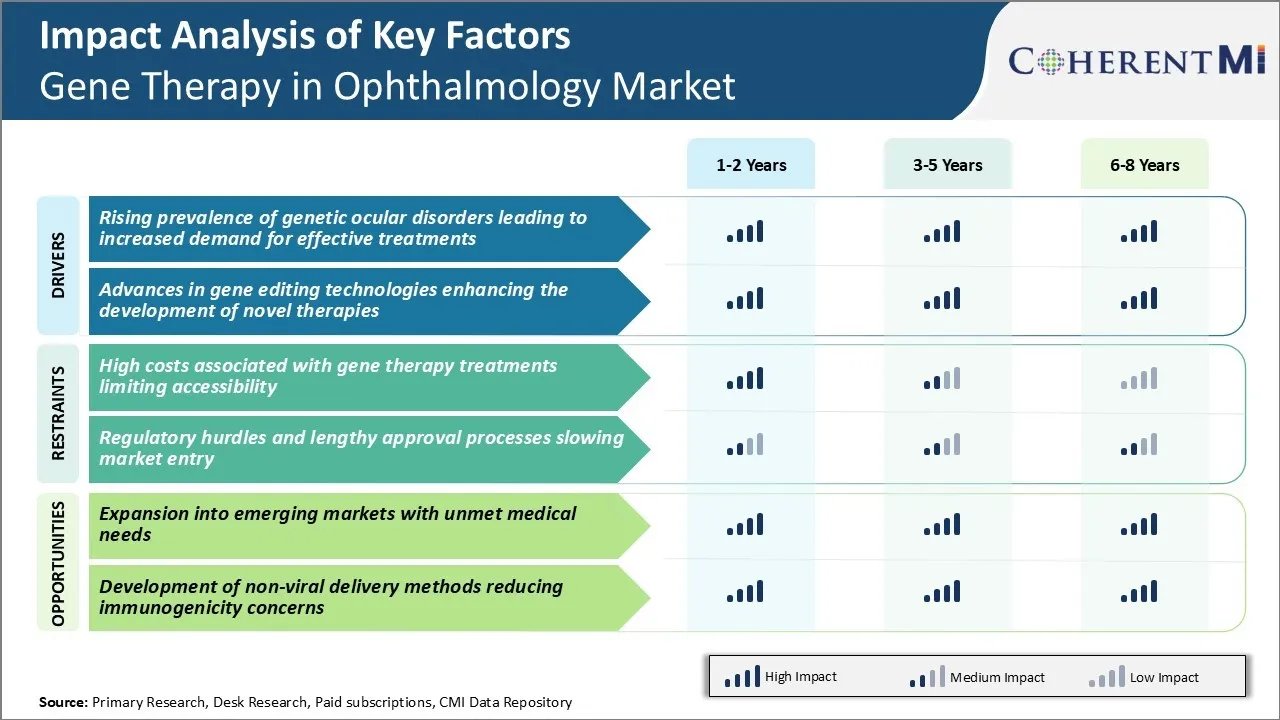
Market Challenge - High Costs Associated with Gene Therapy Treatments Limiting Accessibility
Gene therapy treatments for various ophthalmic diseases have shown tremendous potential in clinical trials. However, one of the major challenges continues to be the high costs associated with developing and delivering such treatments. Developing an effective gene therapy typically requires years of rigorous research and testing. This clinical development process is highly expensive and risky.
Additionally, manufacturing gene therapies is a complex process that often involves customized viral vectors and careful dosing for each patient's specific needs. All of these factors contribute to the high price tags of many gene therapies currently available or in the pipeline.
For example, voretigene neparvovec, the first approved gene therapy for an inherited retinal disease, has a one-time list price of $850,000 per treatment. While it has shown promising results for patients, such high costs limit patient access and insurer reimbursement for this novel treatment approach.
As gene therapies for ophthalmic conditions continue to advance, bringing down the costs associated with development as well as administration will be crucial to maximize the utilization and benefits of this technology across broader patient populations.
Market Opportunity - Expansion into Emerging Markets with Unmet Medical Needs
One significant opportunity for the gene therapy market in ophthalmology lies in expanding into emerging markets across Asia, Latin America, Middle East, and Africa. Many populations in these regions suffer from a high prevalence of blinding ophthalmic diseases but have lacked access to advanced treatment options due to various economic and infrastructure barriers.
Gene therapies have the potential to transform the management of genetically-driven retinal disorders that currently have limited treatment options in these markets. International drug developers and domestic pharma companies can capitalize on this unmet need by conducting clinical trials and regulatory submissions strategically tailored for emerging markets. Partnering with local healthcare providers and patient advocacy groups will help facilitate patient identification and treatment delivery.
A successful emerging market expansion strategy could allow gene therapy companies to access larger patient pools and grow revenues in a meaningful way. This would also help fulfill the goal of making such revolutionary therapies globally accessible.