Global Sialorrhea Treatment Market Size - Analysis
Market Size in USD Mn
CAGR5.5%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 5.5% |
| Market Concentration | High |
| Major Players | Merz Pharmaceuticals, US WorldMeds, NeuroHealing, Proveca, Eisai (via Sloan Pharma) and Among Others |
please let us know !
Global Sialorrhea Treatment Market Trends
With increased awareness about conditions like Parkinson's disease, motor neuron disease, and mental illnesses, the diagnosis of associated symptoms like excessive drooling or sialorrhea has also improved. Healthcare professionals are now able to better identify cases of sialorrhea compared to a few years ago. Several programs aimed at educating doctors as well as the public have played a key role in enhancing the understanding of this uncomfortable symptom. Patients who may have simply ignored excess saliva production in the past are now seeking medical help.
The approval of Botulinum toxin by the US FDA in 2011 was a landmark event in the sialorrhea treatment space. The novel therapy Myobloc, designed to treat chronic sialorrhea, offered patients and physicians an alternative to radiation therapy or surgery. It provided a minimally invasive and relatively well-tolerated option to control excess drooling. This approval inspired increased faith in pharmaceutical innovations and bolstered overall research efforts targeting sialorrhea. It has strengthened industry aspirations to enhance product pipelines and accelerate new launches.
Notwithstanding rare adverse effects, clinicians find Botulinum toxins as reliable treatments for adults and pediatric cases of sialorrhea. Their efficacy has mitigated the requirement for alternative methods and raised hope for discovering safer, targeted options. The therapeutic arrival has fueled momentum in clinical trials exploring other classes of molecules to manage this distressing condition. Patent expirations too will likely stimulate competition and price declines, improving accessibility in the coming years.

One of the key challenges currently facing the sialorrhea treatment market is the high costs of approved treatment options such as botulinum toxin injections. These injections are an effective therapy for reducing saliva production in patients with conditions that cause excessive drooling such as cerebral palsy. However, each treatment session involves injecting minute doses of botulinum toxin into the salivary glands under local anesthesia. As botulinum toxin drugs like Botox and Dysport are biologics derived from living cells, the manufacturing process is complex and costs are high.
One of the key opportunities in the sialorrhea treatment market lies in the ongoing expansion of neuromodulator drugs Xeomin and Myobloc into new geographical markets and patient populations. Xeomin and Myobloc are two alternative botulinum toxin drugs to Botox and Dysport that are currently only approved for treating sialorrhea in a few countries. Both these drugs have demonstrated strong efficacy in reducing drooling with an improved safety profile over older drugs.
Prescribers preferences of Global Sialorrhea Treatment Market
Sialorrhea, or excessive saliva production, is commonly seen in patients with neurologic conditions such as Parkinson's disease, amyotrophic lateral sclerosis, and cerebral palsy. Treatment approaches vary based on the severity and progression of symptoms.
For moderate sialorrhea, anticholinergic drugs that work peripherally are prescribed. Common options at this stage include brand names like Robinul (glycopyrrolate) and Kwells (orphenadrine). These work by reducing saliva secretion from salivary glands.
When medications prove ineffective or intolerable due to severe side effects like mental status changes or constipation, the next option is minimally invasive procedures like intraglandular botulinum toxin injections or radiotherapy. As a last resort, surgery to remove major salivary glands may be considered.
Key winning strategies adopted by key players of Global Sialorrhea Treatment Market
Drug development and innovation: Leading players like Lundbeck, GlaxoSmithKline, and Sun Pharmaceuticals have focused on developing innovative drug formulations and delivery methods for treating sialorrhea. For example, in 2017 Lundbeck received FDA approval for Naldemedine (Rotegrity) tablets for treating opioid-induced sialorrhea. The once-daily oral medication was found to significantly reduce excess saliva production compared to placebo.
Geographic expansion: Market leaders have focused on expanding into high-growth regions like Asia Pacific and Latin America through new approvals, regional manufacturing plants and licensing agreements with local players. For example, between 2013-2018 GSK received aprovals for Glycopyrrolate tablet in over 15 countries, helping them become a top player in global markets.
Segmental Analysis of Global Sialorrhea Treatment Market
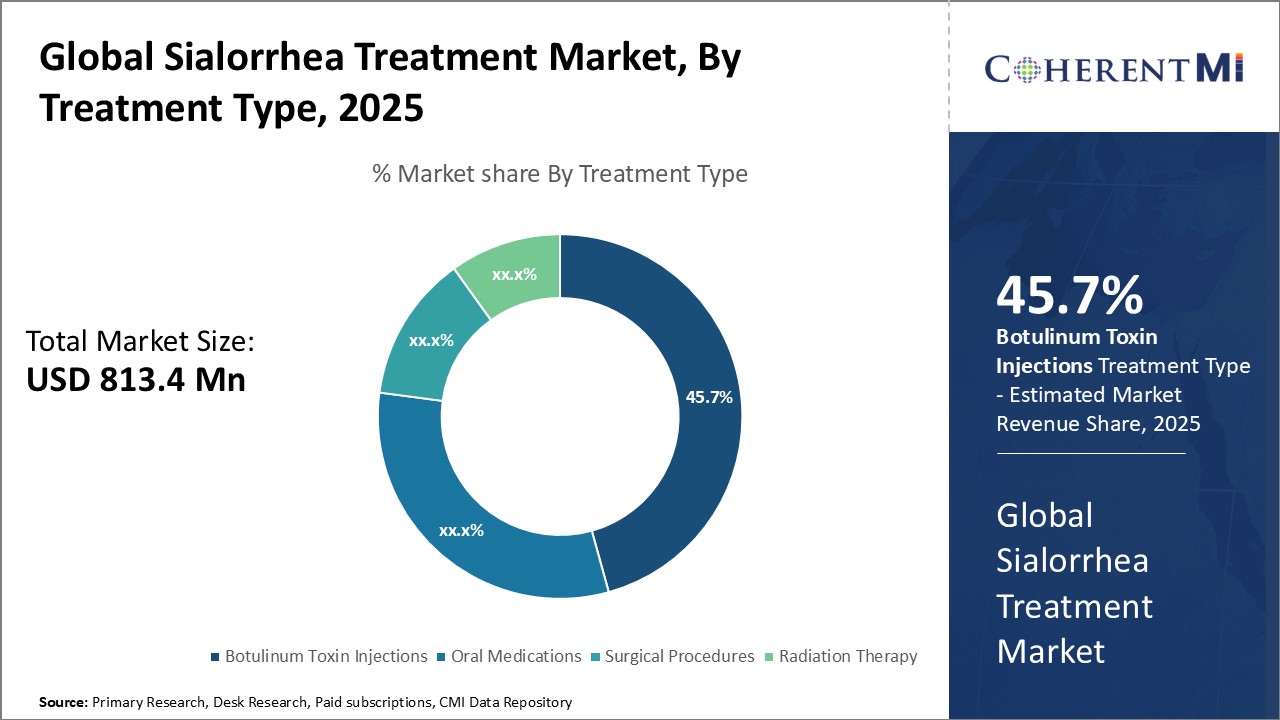
Insights, By Treatment Type: Botulinum Toxin Injections (Xeomin, Myobloc) Draw Attention in the Market
Additionally, botulinum toxin treatments offer advantages in terms of speed of action and reversibility of effects. Results are visible within 2-4 weeks of administration and symptoms typically resolve within 3-6 months as the muscles regain functionality. Meanwhile, effects of surgical gland removal or radiotherapy may persist permanently. The temporary paralytic impact also allows physicians to customize dosage for each patient until optimal symptom relief is achieved. Repeated injections can be administered safely as required.
Additional Insights of Global Sialorrhea Treatment Market
- Myobloc was initially approved for cervical dystonia but later expanded its indication to treat Sialorrhea.
- XEOMIN became the first FDA-approved neuromodulator for treating Sialorrhea in children.
- In 2023, the United States recorded approximately 336,800 cases of chronic Sialorrhea in patients with Parkinson’s disease.
- Germany has the highest prevalence of Sialorrhea in the EU4 countries.
Competitive overview of Global Sialorrhea Treatment Market
The major players operating in the sialorrhea treatment market include Merz Pharmaceuticals, US WorldMeds, NeuroHealing, Proveca, and Eisai (via Sloan Pharma).
Global Sialorrhea Treatment Market Leaders
- Merz Pharmaceuticals
- US WorldMeds
- NeuroHealing
- Proveca
- Eisai (via Sloan Pharma)
Global Sialorrhea Treatment Market - Competitive Rivalry

Global Sialorrhea Treatment Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Global Sialorrhea Treatment Market
- In August 2019, US WorldMeds received FDA approval for Myobloc (rimabotulinumtoxinB) for the treatment of chronic Sialorrhea in adults. This made Myobloc the first FDA-approved botulinum toxin for this condition. Myobloc works by blocking the release of acetylcholine, reducing saliva production, and its effects last up to three months. This approval created significant opportunities in the treatment landscape for Sialorrhea, especially for patients with neurological disorders like Parkinson’s disease
- In December 2020, Merz Pharmaceuticals obtained FDA approval for Xeomin, expanding its use to children aged 2 years and older. This marked an expansion of its use, positioning Xeomin as a treatment for both pediatric and adult patients. This development strengthened Xeomin's standing in the treatment of Sialorrhea.
Global Sialorrhea Treatment Market Segmentation
- By Treatment Type
- Botulinum Toxin Injections (Xeomin, Myobloc)
- Oral Medications
- Surgical Procedures
- Radiation Therapy

Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Frequently Asked Questions :
How big is the sialorrhea treatment market?
The sialorrhea treatment market is estimated to be valued at USD 813.4 Mn in 2025 and is expected to reach USD 1,110 Mn by 2032.
What are the key factors hampering the growth of the sialorrhea treatment market?
The high costs of approved treatments and therapies and limited awareness in some geographical regions are the major factor hampering the growth of the sialorrhea treatment market.
What are the major factors driving the sialorrhea treatment market growth?
The increasing awareness and diagnosis of sialorrhea and approval of novel therapies like myobloc and xeomin are the major factor driving the sialorrhea treatment market.
Which is the leading treatment type in the sialorrhea treatment market?
The leading treatment type segment is Botulinum Toxin Injections (Xeomin, Myobloc).
Which are the major players operating in the sialorrhea treatment market?
Merz Pharmaceuticals, US WorldMeds, NeuroHealing, Proveca, and Eisai (via Sloan Pharma) are the major players.
What will be the CAGR of the sialorrhea treatment market?
The CAGR of the sialorrhea treatment market is projected to be 5.5% from 2025-2032.