Pertussis Therapeutic Market Size - Analysis
The pertussis therapeutic market is estimated to be valued at USD 3.94 Bn in 2025 and is expected to reach USD 5.05 Bn by 2032, growing at a compound annual growth rate (CAGR) of 3.6% from 2025 to 2032. The market has been witnessing steady growth over the past few years driven by high prevalence of pertussis across the globe.
Market Size in USD Bn
CAGR3.6%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 3.6% |
| Market Concentration | High |
| Major Players | Tianjin CanSino Biotechnology, ILiAD Biotechnologies, GlaxoSmithKline (GSK), Sanofi, AstraZeneca and Among Others |
please let us know !
Pertussis Therapeutic Market Trends
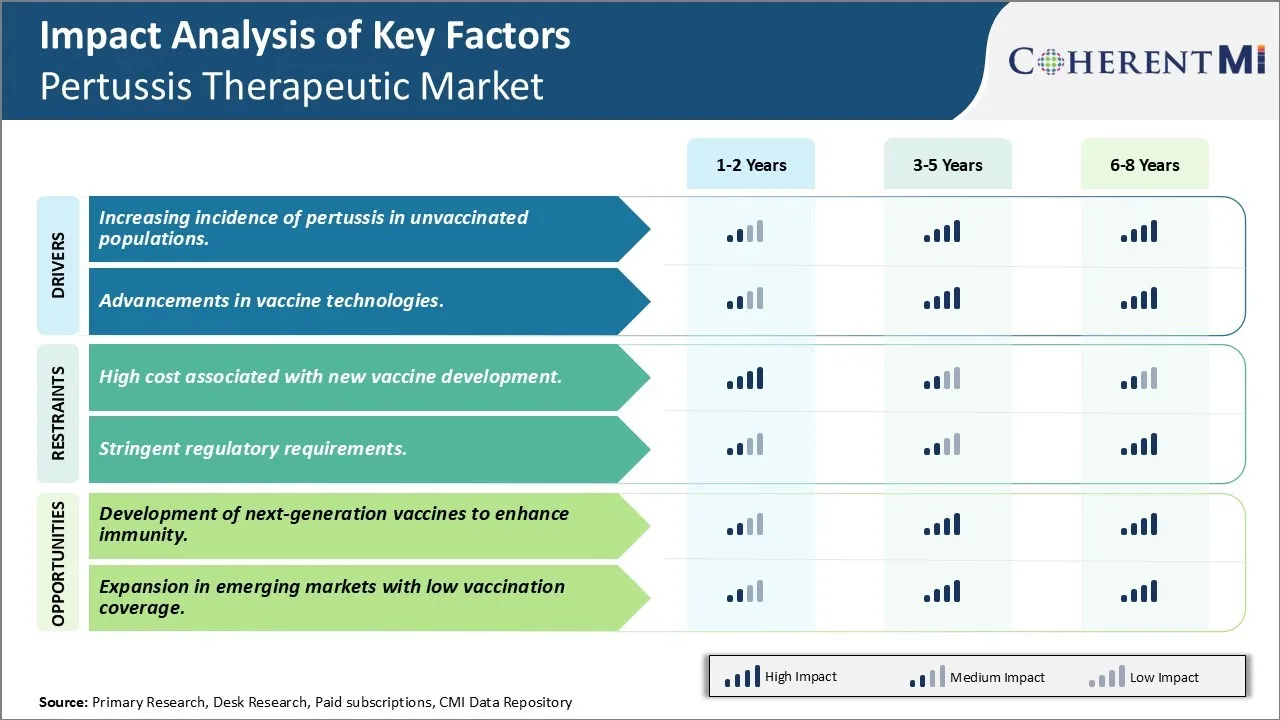
Market Driver - Increasing incidence of pertussis in unvaccinated populations
The resurgence of pertussis or whooping cough in the past decade, especially among unvaccinated populations has been a major concern for global public health experts. While pertussis vaccines have dramatically reduced cases in the late 20th century, recent years have witnessed an increase in reported pertussis cases. This rise has predominantly been among children, teenagers as well as adults who were unvaccinated or whose immunity has waned over time. The heightened vulnerability of unvaccinated populations is driving a potential demand for improved preventive options against this disease.
According to CDC reports, the last pertussis epidemic occurred from 2004–2005 and 2019–2020 has seen a sharp uptick in cases across several parts of United States. Many pediatricians point towards smaller percentage of children receiving timely vaccination as the likely cause. Several developed nations too have reported higher incidence among school going children and teenagers. The World Health Organization data suggests that global circulation of pertussis is widespread and disease transmission remains high in many communities despite vaccine coverage being adequate as per standards.
Market Driver - Advancements in vaccine technologies
Vaccine research has made immense progress in past decades; however pertussis vaccine technology has limitations like transient immunity which needs addressing. Scientists are constantly working towards enhancing vaccine performance. Several innovative vaccine platforms and antigen design strategies are being studied that can expedite the development of next generation pertussis vaccines.
One key area of research is developing acellular vaccines combining genetically engineered pertussis toxin mutants with other antigens. Genetic engineering allows production of non- or less-toxic mutants that are suitable as vaccine components. Combining these with immunostimulants holds promise to enhance the magnitude and quality of immune responses. Adjuvanted recombinant vaccines made of multiple antigen components conjugated to immunopotentiators are anticipated to induce improved protection.
Next-generation approaches like reverse vaccinology based on genomic sequences are utilized for identifying novel antigens. Studies have revealed proteins like BrkA, Vag8/9, FHA2 as potential candidates and various combinations are tested for synergistic effects. Newer technologies like protein structure prediction, in silico antigen design aid selection of epitopes that can pack maximum immunogenic firepower.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - High cost associated with new vaccine development
One of the key challenges faced in the pertussis therapeutic market is the high cost associated with new vaccine development. Developing a new vaccine requires extensive research and clinical trials to ensure safety and efficacy. This vaccine development process involves long periods of research, testing, regulatory review and approvals. It typically takes 10-15 years for a vaccine candidate to progress from discovery to regulatory approval and introduction in the market. Significant investments are required at each stage of vaccine R&D which pushes up the overall costs. The high capital requirements and risk of failure add to the costs. Additionally, the specialized equipment, facilities and skilled researchers required to develop vaccines add to their production costs. Stringent regulations related to vaccine safety and quality assurance further increase compliance costs for manufacturers. Unforeseen delays or regulatory roadblocks during clinical trials or approvals can significantly increase the costs beyond initial estimates. The incurred costs have to be recovered through final vaccine prices making new vaccines expensive for public health programs in resource constrained markets.
Market Opportunity - Development of next-generation vaccines to enhance immunity
One major opportunity in the pertussis therapeutic market is the development of next-generation vaccines that provide enhanced and longer lasting immunity. Currently available whole cell and acellular pertussis vaccines have limited durability of protection with immunity waning within 5-10 years post vaccination necessitating booster doses. There exists a need for vaccines that induce strong and long lasting immune memory against Bordetella pertussis. Novel conjugate, subunit, recombinant, live-attenuated and mRNA based vaccine platforms are being researched that can stimulate both cell mediated and neutralizing antibody immune responses through precision targeting of virulence factors. Advanced vaccine design approaches incorporating multiple antigens, immune potentiators and novel delivery methods hold promise to induce durable protective immune responses after fewer doses or a single dose vaccination. Development of vaccines that provide lifelong immunity or protection beyond childhood could reduce disease burden significantly and cut healthcare costs related to repeated vaccinations. Next-generation pertussis vaccines also provide opportunities for combination vaccines that protect against multiple respiratory illnesses.
Key winning strategies adopted by key players of Pertussis Therapeutic Market
GlaxoSmithKline has been the market leader in pertussis vaccines for decades due to its effective product differentiation strategy. In the 1990s, when efficacy of whole-cell pertussis vaccines started declining, GSK developed the acellular pertussis vaccine called Infanrix. This vaccine used purified pertussis antigens instead of whole killed bacteria. Clinical trials showed Infanrix had a better safety profile with fewer side effects compared to whole-cell vaccines. It received FDA approval in 1996 and quickly captured over 70% of the US pediatric pertussis market.
Sanofi adopted a dual strategy of collaboration and acquisition to strengthen its position. In 2008, it partnered with Biological E to develop an affordable acellular pertussis vaccine for developing countries. This vaccine was launched in India in 2015. Around the same time, Sanofi acquired Protein Sciences Corporation, which had developed an acellular vaccine candidate called SHIP. SHIP showed promise in clinical trials and was acquired by Sanofi to accelerate its development and marketing.
Mitsubishi Tanabe Pharma focused its strategy on the adolescent and adult vaccination market, which was largely untapped until recently. It developed Tdap vaccines like Infanrix IPV and Boostrix that protect against tetanus, diphtheria and pertussis. Clinical trials in Japan showed these vaccines induced strong immune responses and were well-tolerated.
Segmental Analysis of Pertussis Therapeutic Market
 To learn more about this report, Download Free Sample Copy
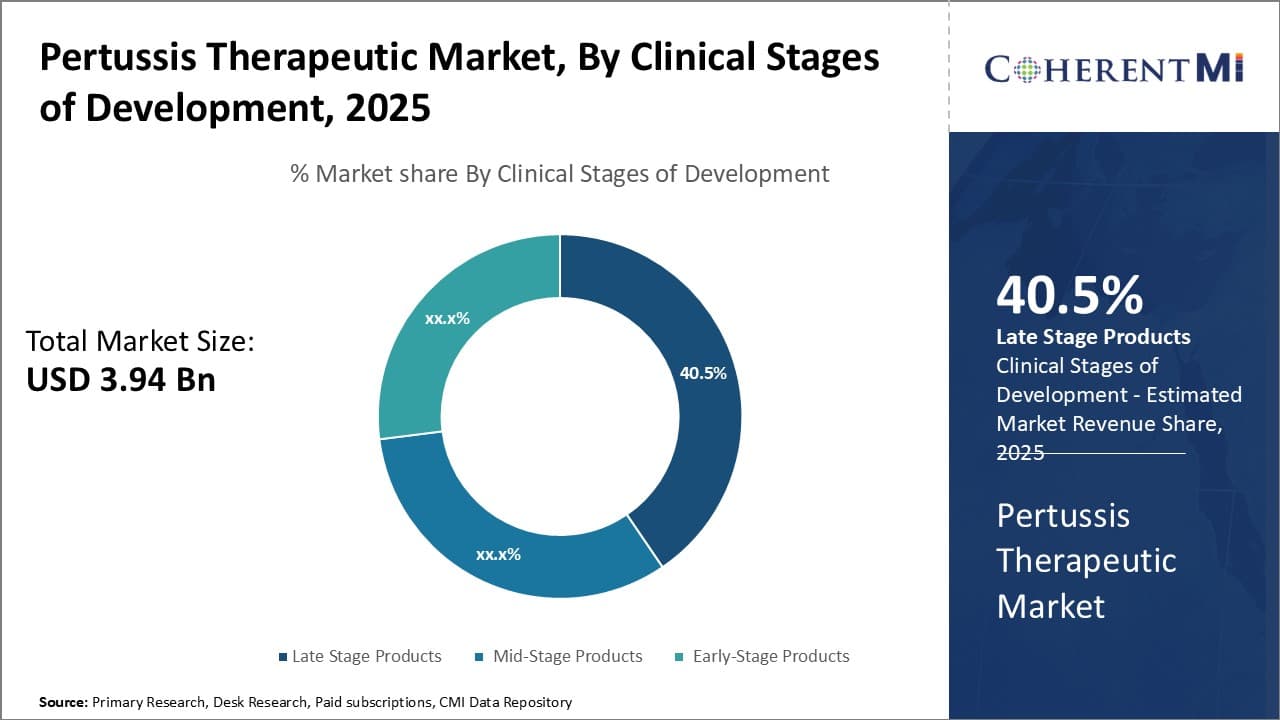
Insights, By Clinical Stages of Development: Late stage products (Phase III) sub-segment contributes the highest share
To learn more about this report, Download Free Sample Copy
Insights, By Clinical Stages of Development: Late stage products (Phase III) sub-segment contributes the highest share
In terms of clinical stages of development, late stage products (Phase III) sub-segment contributes the highest share of 40.5% in the market owing to their proximity to commercialization. Products in late-stage clinical trials have demonstrated safety and efficacy, having passed Phase I and Phase II testing. This significantly de-risks their development timeline and increases the probability of regulatory approval compared to earlier-stage programs.
Companies prioritize advancing their late-stage assets because of this lower risk profile. Significant resources have already been invested in products that have reached Phase III testing. Successfully completing large, late-stage clinical trials and gaining approval represents the potential payoff on these sizable investments. It also means these products are closer to generating revenue from product sales.
The large patient populations enrolled in Phase III tests further refine appropriate positioning, dosing, and maximize understanding of a drug's performance. This late optimization improves the product profile and helps companies communicate a clear value proposition to prescribers and payers. It establishes well-defined commercial and reimbursement strategies prior to approval and launch.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Insights, By Clinical Stages of Development: Oral Administration Dominance
In terms of route of administration, the oral sub-segment contributes the highest share of 20.8% owing to preferences for convenient dosing forms among patients and prescribers. Oral drug delivery provides simplicity compared to options like injections that require syringes or intravenous administration done in clinical settings.
Convenience is a key factor influencing patient access and medication adherence. People are more likely to correctly and consistently take oral medications as directed versus treatments requiring visiting medical facilities or healthcare professionals for administration. This enhances clinical outcomes and lowers healthcare costs from improved adherence and fewer misreported doses.
Oral drugs also empower patients to self-administer therapy as needed, such as promptly starting treatment after potential exposure. This facilitates faster response times compared to treatments reliant on third-party administration. It increases the control patients feel over managing their condition.
From the prescriber perspective, oral medications are also more conformable to recommend long-term for asymptomatic carriers and immune compromised patients at higher risk of Pertussis. Simpler dosing translates to greater prescription volumes driven by the user-friendly nature and reliable outcomes of oral delivery.
Competitive overview of Pertussis Therapeutic Market
The major players operating in the pertussis therapeutic market include Tianjin CanSino Biotechnology, ILiAD Biotechnologies, GlaxoSmithKline (GSK), Sanofi, AstraZeneca, Pfizer, Merck & Co., Mitsubishi Tanabe Pharma, Serum Institute of India, and Dynavax Technologies.
Pertussis Therapeutic Market Leaders
- Tianjin CanSino Biotechnology
- ILiAD Biotechnologies
- GlaxoSmithKline (GSK)
- Sanofi
- AstraZeneca
Recent Developments in Pertussis Therapeutic Market
- In August 2023, Tianjin CanSino Biotechnology initiated Phase III trials for DTcP Infant vaccine. This step is crucial for domestic market entry and reducing dependency on imported vaccines.
- ILiAD Biotechnologies is conducting clinical trials for BPZE1, a next-gen pertussis vaccine, aiming to block the colonization of B. pertussis in nasal passages, reducing transmission and disease incidence.
Pertussis Therapeutic Market Segmentation
- By Clinical Stages of Development
- Late Stage Products (Phase III)
- Mid-Stage Products (Phase II)
- Early-Stage Products (Phase I)
- By Route of Administration
- Oral
- Intravenous
- Subcutaneous
- Parenteral
- Topical
Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
