

The Prader-Willi Syndrome (PWS) market is estimated to be valued at USD 0.98 Bn in 2025 and is expected to reach USD 1.59 Bn by 2032, growing at a compound annual growth rate (CAGR) of 7.2% from 2025 to 2032.
Increase in research funding for developing new and improved treatment options for PWS is a key factor driving the market. Several clinical trials evaluating the safety, efficacy of new treatment approaches such as restriction of calorie intake, growth hormone supplementation, and behavior therapy are currently underway.
Market Size in USD Bn
CAGR7.2%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 7.2% |
| Market Concentration | High |
| Major Players | Soleno Therapeutics, Harmony Biosciences, Pfizer, Novo Nordisk, Sandoz and Among Others |
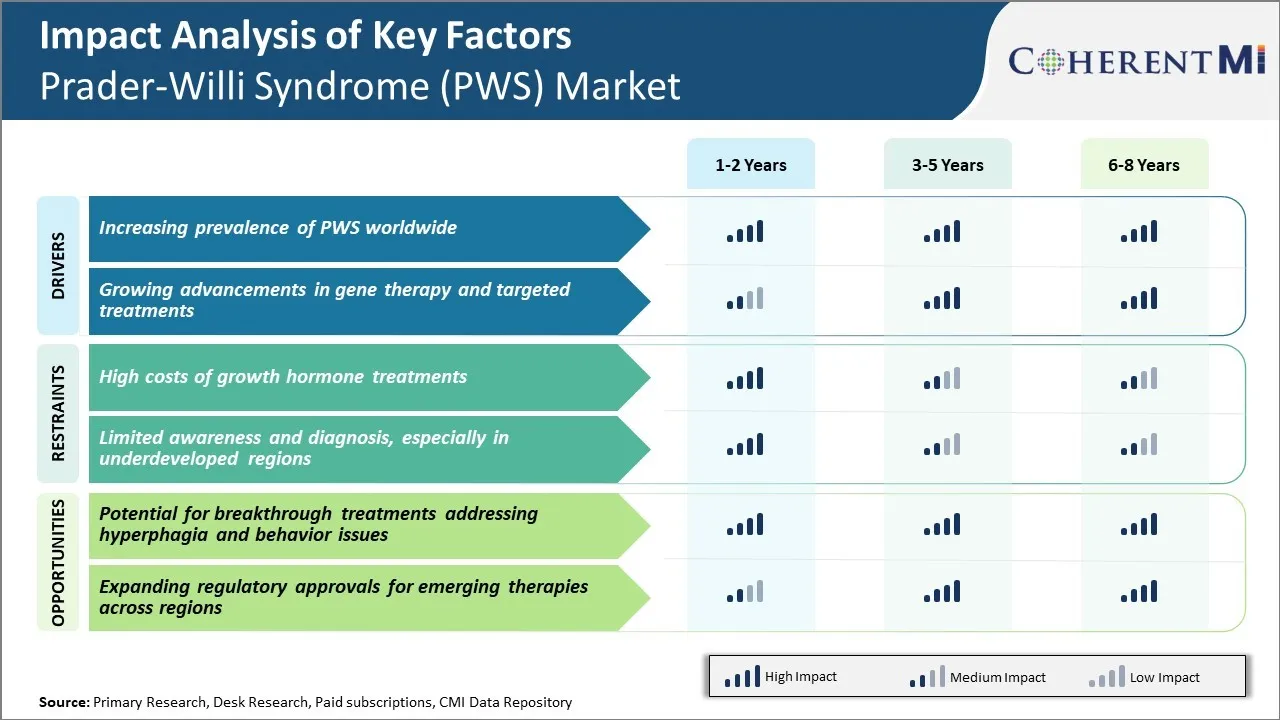
Market Driver - Increasing Prevalence of PWS Worldwide
Prader-Willi Syndrome is a rare genetic disorder that is caused by the loss of specific genetic material on chromosome 15. Though it is still considered a rare condition, analysis suggests that the prevalence of PWS is increasing in different parts of the world. Some of the key reasons for this increase could be better recognition of the syndrome due to more awareness among physicians and general public as well as improvements in diagnostic capabilities.
Today, with advancements in genetic testing and diagnostic methods, more cases are being correctly identified. Additionally, concepts like newborn screening have allowed early diagnosis. This has majorly contributed to observing elevated prevalence figures in the recent years. Studies have also indicated rising prevalence with age which reaffirms this aspect.
Experts feel that as life-expectancy of patients grow due to advance care, the population thriving with PWS will continue expanding over the coming years. Some research reviews have proposed that close to 1 in every 15,000 to 30,000 people worldwide may have PWS.
While still remaining a rare group, the persistent increase in prevalence signifies enhanced diagnosis and longer survival of more individuals impacted by PWS. This highlights the need for continued focus on evolving treatment needs of the patient population.
Market Driver - Growing Advancements in Gene Therapy and Targeted Treatments
Developing innovative treatment approaches has been a major focus for PWS given the complex nature and multi-systemic involvement. Researchers have made remarkable progress especially in the domain of gene therapy and targeted therapies over the last decade.
Ongoing intense research aimed at better understanding the molecular mechanisms of PWS have opened up possibilities that seemed distant not long ago. A host of new treatment candidates are being assessed that hold promise to fundamentally modify the disease phenotype if proven effective and safe.
One significant achievement was recent successful preclinical studies and early-stage human trials evaluating antisense oligonucleotides (ASOs) and other gene silencing techniques. Meanwhile, other modalities in active testing include gene replacement therapy through recombinant adeno-associated virus vectors, genome editing using CRISPR-Cas9 system and miRNA replacement.
Alongside genetic interventions, better characterization of deficiencies in PWS have led to targeted pharmaceutical agents entering evaluation. Research is also looking into drugs focusing on hormonal imbalance, behavioral symptoms, muscular dysfunction and gastrointestinal issues.
Lastly, there is accelerated movement towards combining therapies to achieve maximum benefit from ongoing groundbreaking work across varied scientific domains associated with PWS. Cumulatively, these trends depict an extremely promising future for individuals impacted by this rare disease.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - High Costs of Growth Hormone Treatments
One of the major challenges for the Prader-Willi Syndrome (PWS) market is the high costs associated with growth hormone treatments. Growth hormone therapy is currently the only approved treatment for PWS; however, it requires daily injections over many years which places a huge financial burden on patients and their families.
The costs of the growth hormones themselves are substantial, with an average annual cost being in excess of $30,000-$40,000 USD per patient. This does not include additional costs for regular medical monitoring and appointments that are required during treatment.
As PWS is a rare condition, insurance coverage can sometimes be a challenge to obtain and the out-of-pocket costs after insurance can still be prohibitive for many patients. This high financial barrier limits access to treatment for large segments of the PWS population. In order to expand access and uptake of growth hormone therapy, new pricing and reimbursement models may need to be explored together with pharmaceutical manufacturers and insurance providers.
Market Opportunity - Potential for Breakthrough Treatments Addressing Hyperphagia and Behavior Issues
One significant opportunity for the PWS market lies in developing new treatment options that can address the hyperphagia and behavioral symptoms of the condition. These are two of the most debilitating aspects of PWS that have no approved drug therapies currently.
A treatment that could help curb extreme hunger episodes and regulate behavior would be transformative for patients and families affected by PWS. It could help reduce health risks, improve quality of life, aid in developmental milestones and allow for better social integration.
The unmet needs in these two core symptom areas represent a major market potential if pharmaceutical companies are able to deliver efficacious and well-tolerated therapies through their research pipelines. Promising new targets and compounds are being explored in clinical trials.
A successful treatment breakthrough could capture a sizable portion of the PWS market and help address one of the most challenging aspects of living with this rare condition.
PWS is a complex genetic disorder associated with developmental delays, cognitive disabilities, low muscle tone and an insatiable appetite. Treatment is typically divided into three stages - infant/toddler, childhood and adulthood.
In infants and toddlers, treatment focuses on maintaining proper nutrition and growth. Prescribers commonly recommend formula supplementation such as Similac to prevent weight loss until solid foods are introduced. During childhood, appetite control medications are often prescribed. First-line options include Saxenda/liraglutide (Novo Nordisk) and Xenical/orlistat (Roche), which work by reducing hunger signals. If weight stabilization isn't achieved, second-line drugs including Symlin/pramlintide (AstraZeneca) may be added.
Into adulthood, lifestyle management remains key. However, some patients require stronger appetite suppressants like Belviq/lorcaserin (Eisai) or Qsymia/phentermine and topiramate ER (Vivus). Ensuring access to multidisciplinary healthcare teams that provide guidance on nutrition, behavior modification and pharmacotherapy influences prescribers' choices at this stage. Ongoing genetic counseling is also important for families affected by PWS.
Treatment for PWS aims to manage the core symptoms at each disease stage.
In infants, feeding and nutritional management is critical to prevent failure to thrive. Calorie-dense formulas are recommended to promote weight gain. Appetite stimulants like Megace may be used.
As children grow, behavioral therapy targets hyperphagia and tantrums. Focus is on establishing routines, reinforcing appropriate behaviors, and preventing obsessive food-seeking. Antipsychotics like risperidone (Risperdal) help reduce related behavioral issues.
During pre-teen and teen years, weight management remains a challenge due to declining metabolism and increased appetite. Low-calorie diets combined with physical activity are emphasized. Continued behavioral therapy aids impulse control around food.
As adults, multidisciplinary care involves addressing medical, nutritional, physical, occupational, speech, and mental health needs. Tested programs aim to develop daily living skills and minimize dependence. Growth hormone therapy may aid weight and body composition if started early.
Throughout the lifespan, genetic counseling is recommended for parents due to the inheritance pattern. Early diagnosis is key to improving outcomes via specialized multidisciplinary care targeting the core issues of each disease stage. A coordinated treatment plan can greatly enhance quality of life.
Drug development and approvals: One of the most successful strategies adopted by major players has been investing heavily in research and development to develop novel drugs for PWS. For instance, in 2017, Roche received FDA approval for its drug Ruconest to treat hereditary angioedema (HAE) attacks in children.
Acquisitions: Acquiring companies with promising drug candidates in clinical trials has helped expand product pipelines. In 2019, Chiesi Farmaceutici acquired Ellepause to gain access to its investigational drug EL012484 for non-alcoholic steatohepatitis (NASH). This allowed Chiesi to strengthen its position in liver diseases. Such acquisitions mitigate research risks and fast-track the review/approval process of acquired drugs.
Partnerships and collaborations: Partnering with other players complements strengths and mitigates weaknesses. For example, in 2018, Novo Nordisk partnered with Dicerna for the development of RNAi therapies for undisclosed rare disease targets. This enabled both companies to capitalize on their respective drug development expertise. Collaborations reduce competition and promote data/resource sharing.
Geographic expansion: Expanding into growing pharmaceutical markets like Asia and Latin America has helped increase sales revenues. For instance, in 2016 Roche entered a strategic collaboration with China's Shanghai Junshi Biosciences to develop and commercialize drugs in China. Roche gained access to China's large patient pool and lower costs of clinical trials.
-market-by-growth-hormone-therapy.webp&w=3840&q=75) To learn more about this report, Download Free Sample Copy
Insights, By Growth Hormone Therapy: Growth Hormone Deficiency Leads to High GENOTROPIN Usage
To learn more about this report, Download Free Sample Copy
Insights, By Growth Hormone Therapy: Growth Hormone Deficiency Leads to High GENOTROPIN Usage
In terms of growth hormone Therapy, GENOTROPIN (Pfizer) contributes the highest share of the market owning to the high prevalence of growth hormone deficiency among PWS patients. GENOTROPIN is a recombinant human growth hormone injection used for the long-term treatment of growth failure in children and growth hormone deficiency in adults.
As per studies, around 85-90% of individuals with PWS demonstrate abnormalities in levels of growth hormone, a hormone which is released from the pituitary gland to stimulate growth. This results in short stature and low muscle mass in patients. GENOTROPIN injection aims to address these issues by replacing the missing growth hormone and facilitating growth for children and maintaining lean body mass for adults.
Its extensive use in tackling a core symptom of PWS has enabled GENOTROPIN to gain significant market share over other growth hormone therapies.
-market-by-hyperphagia-treatment.webp&w=3840&q=75) To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Insights, By Hyperphagia Treatment: Hyperphagia Targeting Boosts DCCR Dominance
In terms of hyperphagia treatment, the Prader-Willi Syndrome (PWS) market is segmented into DCCR (Soleno Therapeutics), Carbetocin (ACADIA Pharmaceuticals), ARD-101. Among these segments DCCR (Soleno Therapeutics) contributes the highest share of the market. DCCR is an oral capsule meant for the chronic treatment of hyperphagia and problematic behaviors among PWS patients.
Hyperphagia, an uncontrollable hunger that can cause extreme obesity, is one of the key challenges in PWS management. DCCR acts as a selective inhibitor of gastric release of ghrelin, a hormone that triggers eating. By mitigating excess ghrelin levels, DCCR demonstrates potential in curbing hyperphagia in these patients. This unique mechanism of action targeting the core cause of hyperphagia has given DCCR an edge over other competitors and driven its segment leadership.
Insights, By Behavioral and Sleep Management: WAKIX Gains Traction for Sleep Issues Treatment
In terms of behavioral and sleep management, the Prader-Willi Syndrome (PWS) market is segmented into WAKIX (Harmony Biosciences). Among these segments WAKIX (Harmony Biosciences) contributes the highest share of the market. WAKIX is the first and only approved treatment indicated for excessive daytime sleepiness and cataplexy in adult patients with narcolepsy. It works by selectively inhibiting orexin receptors, neuropeptides responsible for regulating wakefulness and REM sleep.
Research unveils a high prevalence of sleep disturbances like daytime drowsiness and sleep apnea among PWS patients as well. With a novel mechanism addressing sleep issues, WAKIX has been able to clinch a strong patient base and lead this segment by providing a much-needed therapeutic solution for behavioral and sleep management in PWS.
The major players operating in the Prader-Willi Syndrome (PWS) market include Soleno Therapeutics, Harmony Biosciences, Pfizer, Novo Nordisk, Sandoz, ACADIA Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, and ConSynance Therapeutics.
Would you like to explore the option of buying individual sections of this report?
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Prader-Willi Syndrome (PWS) Market is segmented By Growth Hormone Therapy (GENOTROPIN, NORDITROPIN, ...
Prader-Willi Syndrome (PWS) Market
How big is the Prader-Willi Syndrome (PWS) market?
The Prader-Willi Syndrome (PWS) market is estimated to be valued at USD 0.98 Bn in 2025 and is expected to reach USD 1.59 Bn by 2032.
What are the key factors hampering the growth of the Prader-Willi Syndrome (PWS) market?
The high costs of growth hormone treatments and limited awareness and diagnosis, especially in underdeveloped regions are the major factors hampering the growth of the Prader-Willi Syndrome (PWS) market.
What are the major factors driving the Prader-Willi Syndrome (PWS) market growth?
The increasing prevalence of PWS worldwide and growing advancements in gene therapy and targeted treatments are the major factors driving the Prader-Willi Syndrome (PWS) market.
Which is the leading Growth Hormone Therapy in the Prader-Willi Syndrome (PWS) market?
The leading growth hormone therapy segment is GENOTROPIN (Pfizer).
Which are the major players operating in the Prader-Willi Syndrome (PWS) market?
Soleno Therapeutics, Harmony Biosciences, Pfizer, Novo Nordisk, Sandoz, ACADIA Pharmaceuticals, Aardvark Therapeutics, Gedeon Richter, Palobiofarma, ConSynance Therapeutics are the major players.
What will be the CAGR of the Prader-Willi Syndrome (PWS) market?
The CAGR of the Prader-Willi Syndrome (PWS) market is projected to be 7.2% from 2025-2032.