Refractory Epilepsy Market Trends
Market Driver - Increasing Prevalence of Drug-Resistant Epilepsy Among Patients Leading to a High Demand for Innovative Treatments
A major driver for the refractory epilepsy market is the rising prevalence of patients who do not respond to conventional antiepileptic drug treatment options. Approximately one third of all epilepsy patients suffer from drug-resistant forms of the condition where seizures are not adequately controlled by two or more tolerated, appropriately chosen and administered antiepileptic drug regimens. If a patient's epilepsy continues to be uncontrolled or cannot be managed by pharmacological interventions, they are classified as having refractory or drug-resistant epilepsy. The underlying causes behind treatment failure vary between patients and can be due to genetic predispositions or structural abnormalities in the brain that prevent drugs from taking full effect. As physicians exhaust conventional treatment options, there is a growing need for novel disease-modifying therapies that target the specific pathophysiological mechanisms responsible for medically intractable seizures in different patient cohorts. Some patients with severe epilepsy that is not controlled with medications may be offered alternative treatment options like neurostimulation therapies or neuromodulation devices which help relieve seizures by delivering electrical signals to specific areas of the brain. The high unmet need to control seizures and improve quality of life for those living with uncontrolled epilepsy is a major factor driving investment into innovative drug and device-based approaches.
Market Driver: Ongoing Research into New Therapeutic Approaches Such as Gene Therapy and Neurostimulation Techniques
Continued research efforts into the development of next-generation therapeutic approaches for refractory epilepsy also serves as an important driver for market growth. Gene therapy is an emerging area of focus that seeks to introduce genetic material into brain cells through viral vectors in order to compensate for genetic mutations linked to certain epilepsies or restore normal neuronal functioning. Early clinical evidence suggests this may help reduce seizure frequency over time. Another avenue being explored is personalized brain stimulation techniques like responsive neurostimulation which monitors brain activity to detect abnormal patterns that precede seizures and then delivers electrical pulses preemptively to interrupt seizures. Device manufacturers are conducting trials of closed-loop stimulation systems that can actively sense seizure onset and intervene appropriately in a closed feedback loop. In addition, transcranial magnetic stimulation is an non-invasive modality gaining ground as a potential treatment for refractory epilepsy either as a stand-alone therapy or complementary intervention used alongside drug regimens. Continued advancements in elucidating disease pathways combined with the commitment of industry players and research organizations to translate findings into scalable therapies will expand available treatment options beyond conventional regimens in the foreseeable future.
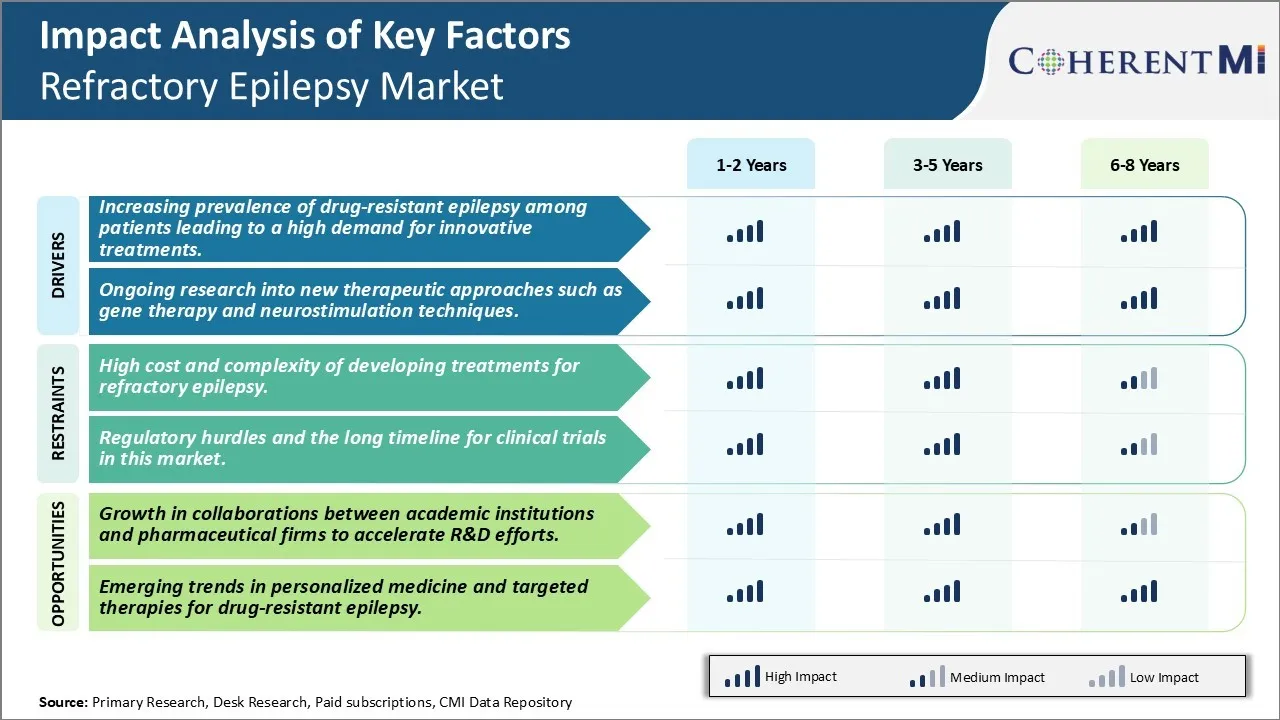
Market Challenge - High Cost and Complexity of Developing Treatments for Refractory Epilepsy
High cost and complexity of developing treatments for refractory epilepsy. Developing new treatments for refractory epilepsy poses significant challenges for pharmaceutical companies. Refractory epilepsy is a complex condition with multiple underlying causes and manifestations. This complexity makes it incredibly difficult for researchers to determine the exact pathological mechanisms and identify effective molecular targets for new drug therapies. Extensive clinical trials are required to test new compounds on refractory epilepsy patients whose conditions may vary dramatically. With each trial costing hundreds of millions of dollars, the financial investment required to bring a new refractory epilepsy treatment to market is extremely high. Additionally, the failure rate of epilepsy drug trials is disproportionately high compared to other therapeutic areas. This increased risk means pharmaceutical companies must accurately assess whether a potential new treatment is likely to succeed before committing massive resources to its development. The molecular heterogeneity of refractory epilepsy combined with the costs and failure risks make developing new therapies an arduous and uncertain endeavour.
Market Opportunity: Partnerships and Collaborations to Create New Avenues for Market
Growth in collaborations between academic institutions and pharmaceutical firms to accelerate R&D efforts. There are signs that this challenging landscape may be improving through innovative partnerships between different sectors. Pharmaceutical companies increasingly recognize that they cannot tackle the complexity of refractory epilepsy alone and are actively collaborating with academic experts. These collaborations pair the pharmaceutical industry's development expertise and financial resources with the basic science knowledge held within academic neurology departments and epilepsy research centres. By combining complementary strengths, collaborations aim to advance understanding of disease mechanisms, identify promising biomarkers and molecular targets, design smarter clinical trials, and translate cutting-edge research into new treatment options more efficiently. If successful, these multi-sector partnerships have the potential to dramatically reduce both the time and costs required to progress refractory epilepsy drug development. Increased collaboration across industry and academia may help address the market's historical difficulties and thus represents an opportunity for accelerated progress towards improved refractory epilepsy treatments.