Triple Negative Breast Cancer (TNBC) Treatment Market Trends
Market Driver - Increasing Incidence of Triple Negative Breast Cancer Globally
The incidence of triple negative breast cancer has been steadily rising across the world in the past few decades. Several epidemiological studies have found higher rates of triple negative breast cancer in younger women less than 50 years of age as compared to other types of breast cancer. Younger women also have poorer survival rates due to more aggressive disease biology and lack of effective targeted therapies.
Furthermore, developing nations are witnessing disproportionately higher incidences particularly among African, Hispanic and Native American women populations who are predisposed to early onset of breast cancer. Experts believe genetic as well as environmental/lifestyle factors like obesity, lack of physical activity, unhealthy diet, alcohol consumption etc. negatively impact the disease risk in these demographics over time.
A recent study estimated over 260,000 new cases and over 75,000 deaths from triple negative breast cancer in 2020 across 184 countries. Global incidences are projected to increase manifold in the coming decades due to above population trends, highlighting the urgent need for development of more effective treatment approaches. This growing patient pool diagnosed with aggressive triple negative breast cancer represents a significant commercial opportunity for novel drugs targeting this high unmet medical need.
Market Driver - Advancements in Immunotherapy and Targeted Drug Delivery Technologies
Scientific advancements in complex fields of cancer immunotherapy and targeted drug delivery have opened promising new avenues for more effective treatment of triple negative breast cancer. Researchers are actively developing diverse modalities to leverage body's natural immune defenses as well as techniques to selectively transport anti-cancer therapies intracellularly.
Cancer immunotherapy relies on enabling the immune system to recognize and destroy cancer cells on its own. Several immunotherapies like checkpoint inhibitors, cancer vaccines, adoptive cell transfer etc. have shown success against other malignancies. Ongoing clinical research is exploring applications of these approaches either as monotherapy or in combination with chemotherapy for TNBC patients. Early results indicate immunotherapy may improve survival when used adjunctively with standard of care regimens. Additionally, factors like high tumor mutational burden in TNBC create an immuno-stimulatory microenvironment more susceptible to immunotherapy interventions.
Research combining antibody-drug conjugates, inorganic nanoparticles, liposomes and other nanocarriers with chemotherapy or targeted agents holds promise. Overall, rapid progress in cancer immunology and nanomedicine is fueling clinical innovation and translational research to design more targeted and well-tolerated TNBC treatment regimens. This is expected to positively impact the triple negative breast cancer (TNBC) treatment market with new therapeutic alternatives in the coming years.
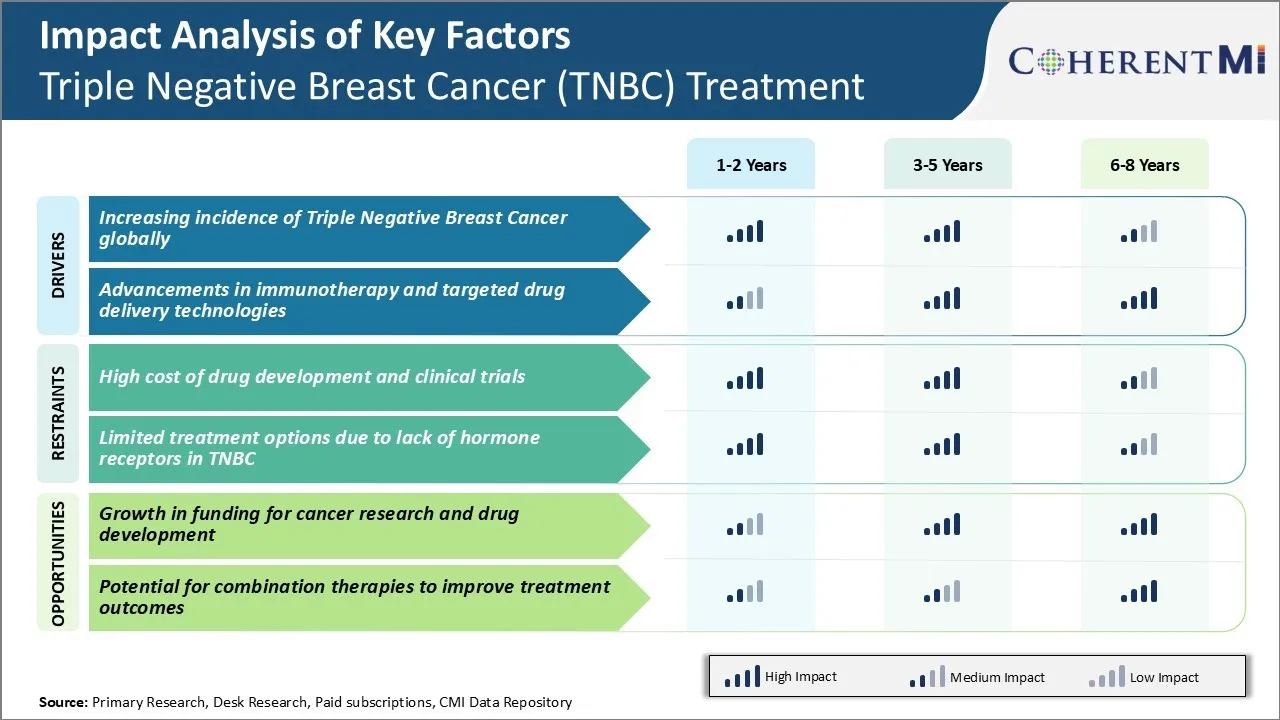
Market Challenge - High Cost of Drug Development and Clinical Trials
One of the major challenges faced by companies operating in the triple negative breast cancer (TNBC) treatment market is the extremely high cost associated with drug development and clinical trials. Developing an entirely new drug from the research stage to final FDA approval typically costs over USD 2 billion.
Specific to TNBC treatments, the development process is further complicated given the heterogeneity and lack of well-defined biomarkers for the disease. This necessitates very large phase 3 clinical trials involving thousands of patients to demonstrate efficacy of an experimental drug. Such late-stage trials alone can cost over $300-500 million per study. Additionally, the failure rate of oncology drugs in phase 3 is as high as 90%, resulting in billions of dollars of losses for companies.
Given the relatively small addressable patient pool for TNBC compared to other breast cancer subtypes, recouping such enormous R&D investments through product sales remains a major challenge. This high-risk, high-cost dynamic has prevented many large pharma players from actively pursuing the triple negative breast cancer (TNBC) treatment market.
Market Opportunity – Growth in Funding for Cancer Research and Drug Development
One key opportunity in the TNBC treatment market is the significant rise in funding availability for cancer research and drug development over the past decade. Both public and private organizations have substantially increased their financial support for advancing scientific understanding of diseases like TNBC and developing novel therapies.
For instance, leading cancer charitable foundations like Breast Cancer Research Foundation and Susan G. Komen have increased their annual research grants to over $100 million collectively. There has also been a parallel surge in venture capital and private equity funding flowing into early-stage oncology biotechs. Such non-dilutive capital has allowed many small companies to advance more TNBC drug candidates into clinical trials.
Additionally, the National Cancer Institute now allocates around $6 billion annually towards cancer research. With ongoing focus on Precision Oncology and targeted therapies, TNBC which lacks well-defined biomarkers is garnering significant funding attention. This availability of capital will help drive further innovation in the triple negative breast cancer (TNBC) treatment market.