United States of America Suboxone Market Size - Analysis
The United States of America Suboxone Market is estimated to be valued at USD 2.39 Bn in 2025 and is expected to reach USD 3.21 Bn by 2032, growing at a CAGR of 4.3% from 2025 to 2032.
Factors such as rising opioid abuse & addiction rates, growth in awareness about drug treatment, availability of generic formulations and favorable reimbursement policies have been driving the market.
Market Size in USD Bn
CAGR4.3%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 4.3% |
| Market Concentration | Medium |
| Major Players | Dr. Reddy’s Laboratories Ltd. , Mylan N.V., Novartis AG, Sun Pharmaceutical Industries Ltd, Alkem Labs and Among Others |
please let us know !
United States of America Suboxone Market Trends
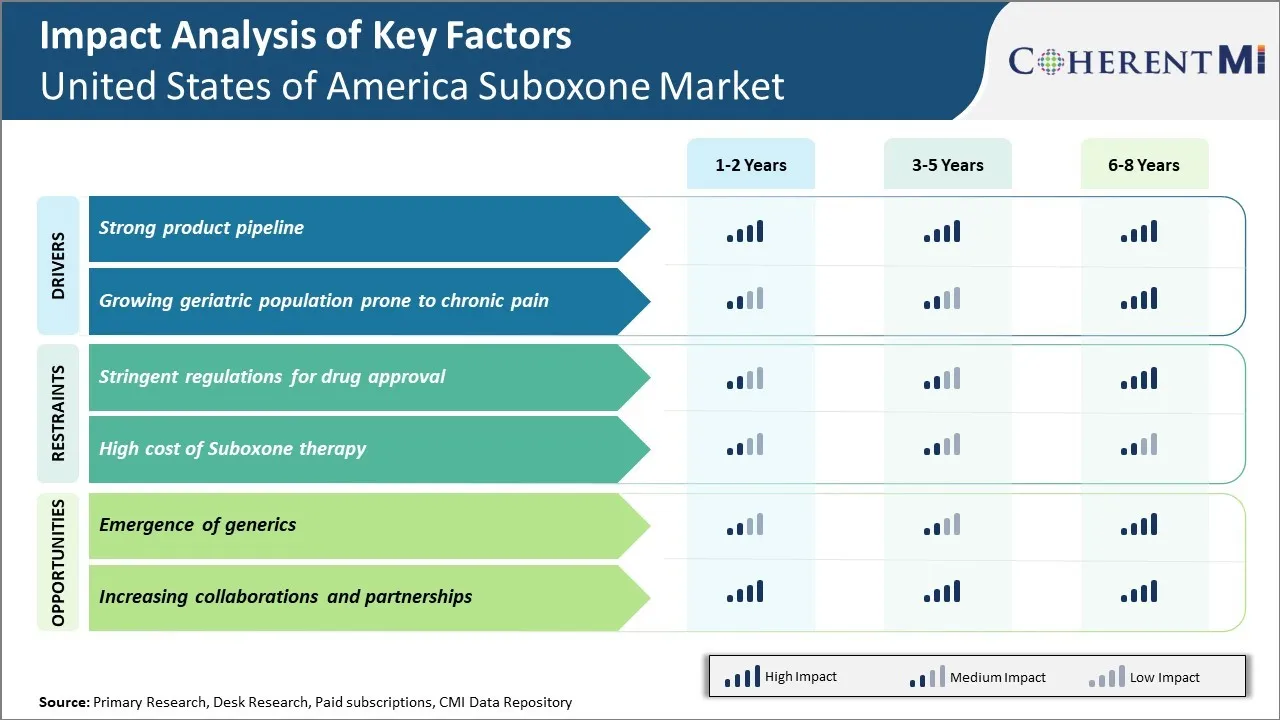
Market Driver – Strong Product Pipeline
The stable and strong pipeline of products in the Suboxone market in the United States of America is a key driver of growth. Companies in the market have consistently introduced new and innovative formulations of Suboxone to treat opioid addiction more effectively.
For instance, in 2022 Indivior, a major player in the market, received FDA approval for Sublocade Monthly (Brixadi), the first long-acting injectable buprenorphine formulation for moderate to severe opioid use disorder. This new product allows for less frequent dosing compared to existing sublingual formulations, increasing treatment convenience and compliance. Other companies like Rhode Pharma have received approval for similar long-acting injections as well as sublingual films with abuse-deterrent properties during the period 2020-2022.
The product pipeline in the upcoming years also remains robust with several pharmaceutical giants like Indivior and Rhodes actively researching newer advanced treatments which are safer and more comfortable for patients. Some formulations under development target specific patient populations like adolescents or allow for monthly or quarterly dosing depending on the individual's needs.
Market Driver – Growing Geriatric Population Prone to Chronic Pain
The growing geriatric population prone to chronic pain is a key driver boosting the United States of America Suboxone market. As per the data from the United States Census Bureau, the population aged 65 years and above is projected to grow considerably in the coming years reaching nearly 94 million Americans by the year 2060. Chronic pain is quite prevalent among the elderly population with over 50% of those aged above 65 facing chronic pain conditions on a daily basis. Some of the most common chronic pain issues experienced by seniors include arthritis, lower back pain, neck pain and overall muscle aches.
This rising prevalence of chronic pain among the aging population is fueling the demand for effective pain management solutions like suboxone in the country. As conventional treatments such as opioids carry risks of dependence and addiction among the elderly, doctors are increasingly recommending buprenorphine-based treatments such as suboxone to geriatric patients experiencing chronic pain. Suboxone offers long-lasting relief from moderate to severe pain and has lesser abuse potential and side effects compared to opioids.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge – Stringent Regulations for Drug Approval
Stringent regulations for drug approval in the United States has greatly impacted the growth of the Suboxone market over the past few years. The drug approval process carried out by the United States Food and Drug Administration (FDA) is an extensive one aimed at ensuring safety and efficacy of new drugs. However, this lengthy and meticulous review cycle has significantly delayed the entry of new and potentially more effective drugs in the suboxone market.
On an average, it takes over 10 years for a new drug to be approved by the FDA from the time clinical trials begin. The drug has to pass through multiple phases of clinical trials involving thousands of volunteers to establish safety and effectiveness for its intended use. Even after successful clinical trials, the FDA conducts a very thorough review of the manufacturing practices and quality control processes before granting final approval. This intense scrutiny is necessary but it extends the time to market for innovator drugs.
Market Opportunity – Emergence of Generics
The emergence of generic versions of Suboxone in the United States represents a significant opportunity for increased access and affordability of medication-assisted treatment for opioid use disorder. For many years, the brand name formulation held a monopoly due to FDA exclusivity protections. However, as of late 2020 the first generic versions of Suboxone sublingual film were approved by the FDA and entered the market. This introduced much-needed competition which has already begun to lower the cost of this vital treatment significantly.
According to recent data from the U.S. Department of Health and Human Services, in 2021 over 9.3 million American adults had a substance use disorder related to pain relievers or heroin. The opioid crisis continues to devastate communities across the country with over 100,000 drug overdose deaths reported in the last year alone. Expanding access to proven treatments such as buprenorphine using generics can help curb this crisis by making treatment more affordable and attainable for those struggling with addiction.
Segmental Analysis of United States of America Suboxone Market
 To learn more about this report, Download Free Sample Copy Insights, By Type: Generics Dominate the Type Segment Due to Lower Prices
To learn more about this report, Download Free Sample Copy Insights, By Type: Generics Dominate the Type Segment Due to Lower Prices
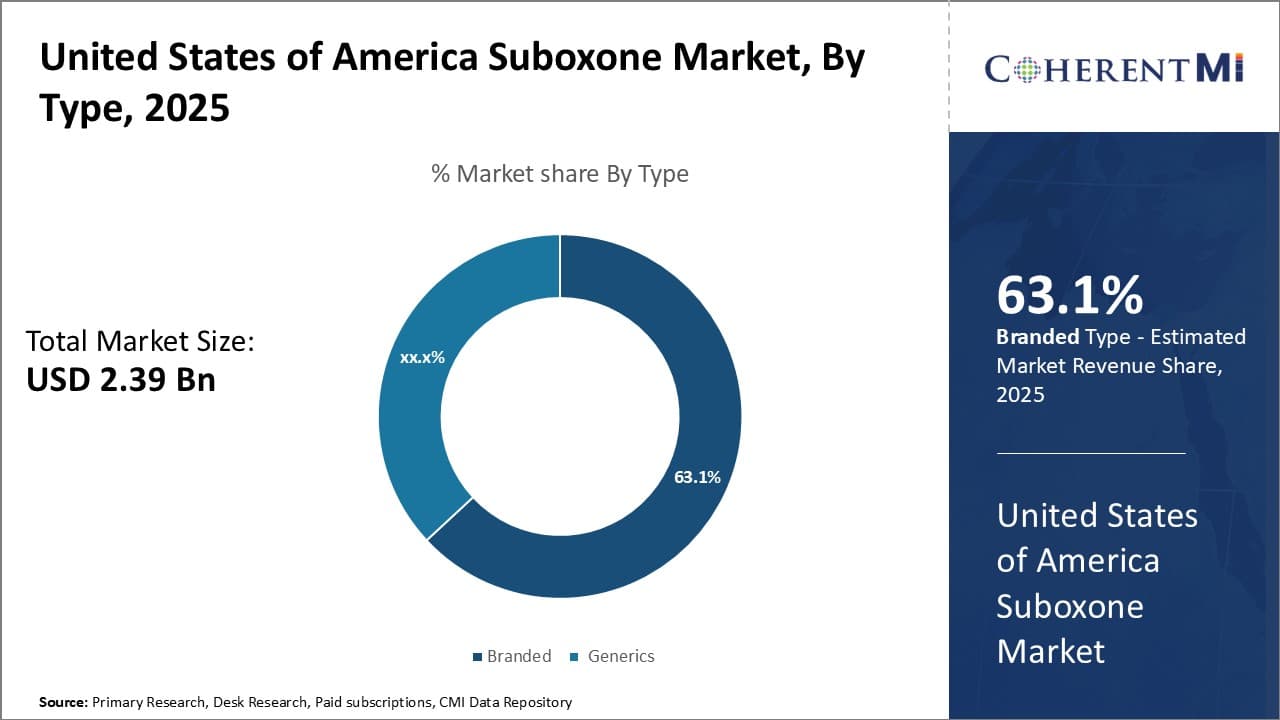
Within the United States of America Suboxone market segmented by type, generics sub-segment contributes the largest share of 63.1%. The primary driver of generics' dominance is their significantly lower prices compared to branded Suboxone. Generics are able to offer prices that are a fraction of the cost of branded drugs once they come off patent exclusivity and face competition. For patients struggling with opioid addiction and seeking treatment, the lower price of generics makes them much more accessible and affordable as an option.
In addition, generics face little resistance from physicians and pharmacies once proven to be just as safe and effective as their branded counterparts. With no difference in clinical performance, generics are freely substituted when presented with a prescription for the branded drug. Widespread acceptance and automatic substitution drive strong market uptake of new generic launches.
Major generic manufacturers like Teva, Mylan, and Sandoz have capitalized on the patent cliff of branded Suboxone to quickly gain market share. Their manufacturing and distribution infrastructure allows nationwide availability, further cementing generics in the top spot. PBM formularies also strongly incentivize the use of low-cost generics through favorable co-pay tier placement and coverage restrictions on higher priced brands.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
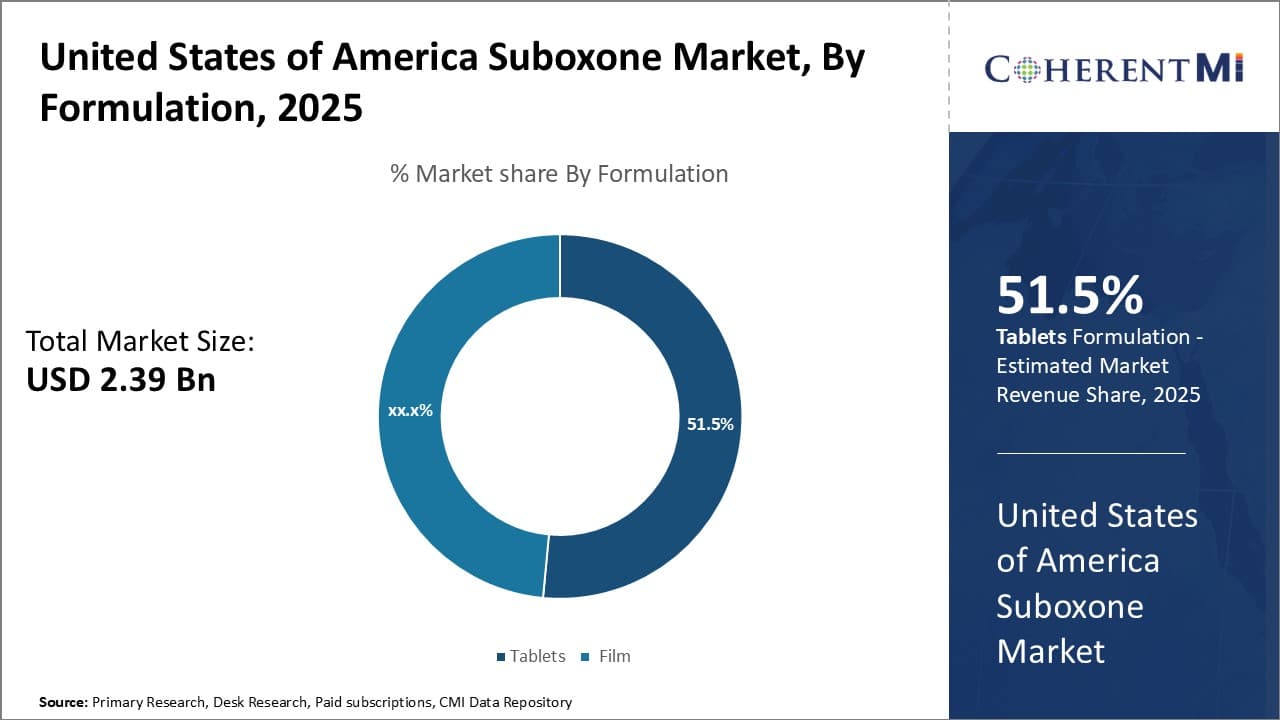
Insights, By Formulation: Simpler and More User-Friendly Dosage Form
When analyzing the United States of America Suboxone market segmented by formulation, tablets contribute the highest share of 51.5% over film. The primary driver of tablets' majority position is their simpler and more user-friendly dosage form compared to film. For those new to treatment or early in their recovery journey, taking a tablet is a much more straightforward process than properly applying and dissolving a small film under the tongue.
Tablets are also generally preferred by physicians just starting patients on buprenorphine-based treatment. The delivery is more tolerant of variations in administration technique compared to film. This provides a gentler onboarding experience as patients master consistent daily dosing. Tablets are also favored in certain populations like older patients who may face more difficulty or have less experience using dissolvable films.
From a supply chain perspective, tablets are simpler and less expensive to manufacture at scale versus film, leading to frequent drug shortages of the latter. Tablet blister packs also take up less physical space on pharmacy shelves compared to bulky film pouches. As a result of these various advantages, tablets have maintained their majority despite the entrance of alternative film offerings in recent years.
Competitive overview of United States of America Suboxone Market
The major players operating in the United States of America Suboxone Market include Indivior PLC., Dr. Reddy’s Laboratories Ltd., Amneal Pharmaceuticals LLC., Mylan N.V., Novartis AG, Teva Pharmaceutical Industries Ltd., Mallinckrodt, Lannett Co Inc., Sun Pharmaceutical Industries Ltd., and Alkem Labs are the major players.
United States of America Suboxone Market Leaders
- Dr. Reddy’s Laboratories Ltd.
- Mylan N.V.
- Novartis AG
- Sun Pharmaceutical Industries Ltd
- Alkem Labs
Recent Developments in United States of America Suboxone Market
- In 2020, the U.S. Food and Drug Administration (FDA) approved a generic drug buprenorphine and naloxone tablet developed by Rhodes Pharmaceuticals, a subsidiary of Purdue, an American privately held pharmaceutical company.
United States of America Suboxone Market Segmentation
- By Type
- Branded
- Generics
- By Formulation
- Tablets
- Film
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Would you like to explore the option of buying individual sections of this report?
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
