산성 Sphingomyelinase 부족 (ASMD) 가격표 규모 및 점유율 분석 - 성장 추세 및 예측 (2024 - 2031)
산성 Sphingomyelinase 부족 (ASMD) 시장은 치료 (XENPOZYME (olipudase alfa), 다른 효소 보충 치료 (ERT)), 지리 (북미, 라틴 아메리카, 아시아 태평양, 유럽, 중동 및 아프리카)에 의해 구분됩니다. 보고서는 상기의 세그먼....
산성 Sphingomyelinase 부족 (ASMD) 가격표 트렌드
시장 드라이버 - ASMD의 Prevalence 성장, 인식과 진단을 증가
ASMD는 산성 sphingomyelinase 효소의 부족에서 결과로 SMPD1 유전자의 mutations에 기인한 극단적으로 희소한 저장 질병입니다. 이 유전적 결함은 sphingomyelin의 정상적인 고장을 방지하고, 세포의 막에서 찾아낸 지방 물질은, 그 때 유해한 수준에 축적하기 위하여 그것을 일으키는 원인이 되었습니다.
역사적으로, ASMD는 전세계 50 ~ 150 명의 알려진 환자의 추정을 가진 가장 희소성 질병 중 하나로 간주됩니다. 그러나, 새로운 연구는 실제적인 우선권이 이전보다 더 높을 수 있습니다 개선 된 진단으로 더 정확하게 식별 할 수 있습니다. 또한, 상태는 심각한 유머 형태와 더 온화한 늦은 온세트 모양 둘 다에 존재한다는 것을 인식됩니다.
ASMD의 진실한 우선권은 더 나은 초점으로 옵니다, 노력은 의학 공동체 및 공중 사이 상태에 관하여 인식을 올리기 위하여 진행됩니다. 환자 옹호 그룹은 징후, 증상 및 사용 가능한 테스트 옵션에 대한 교육에 중요한 역할을합니다. 전통적으로 침략적인 뼈 marrow biopsy에 의존하는 진단 그러나 새로운 건조한 혈액 반점 시험은 더 편리한 신생아 검열 및 늦게에 환자의 진단을 허용합니다. 진단 더 쉬운 통로와 결합된 전반적인 인식을 증가시킨 것은 전 세계 ASMD 케이스의 ID 그리고 확인을 촉진합니다.
시장 드라이버 - XENPOZYME의 FDA 승인 및 미국 및 유럽의 강력한 시장 수요
ASMD 치료 시장 내의 또 다른 키 드라이버는 XENPOZYME의 FDA의 최근 승인이며,이 드문 유전 장애에 특히 표시된 최초의 약물입니다. Genzyme에 의해 개발, Sanofi 회사, XENPOZYME는 1 월 2022에서 가속 된 승인을 받았다 데이터는 세포의 sphingomyelin 축적을 감소시키기 위해 그것의 능력을 해독. 그것은 재조합 인간 산 sphingomyelinase 효소 보충 치료는 항염증제 또는 누락한 효소를 대체하기 위하여 예정된 IV 주입 또는 주입으로 주간을 관리했습니다.
XENPOZYME는 이전에 symptomatic 배려를 통해서 전적으로 관리된 ASMD를 위한 첫번째 질병 대우 선택권을 대표합니다. Clinicians는이 진보적 인 상태의 종종 검증 멀티 시스템 합병에 영향을 미치는 잠재력을 인식합니다. 초기의 실제 경험은 임상 시험에서 입증된 긍정적인 효능과 안전성을 지원합니다.
환자와 의사 설문 조사에 초점을 맞춘 시장 조사는 XENPOZYME는 전세계 ASMD에 대한 치료의 표준이 될 것입니다. 전반적으로, 이 획기적인 표적 치료의 가용성은 중요한 성장 운전사이기 위하여 예상되고, 진단 비율을 증가하고 앞으로 년에 있는 전반적인 산 sphingomyelinase 부족 (ASMD) 시장 가치를 연료를 공급합니다.
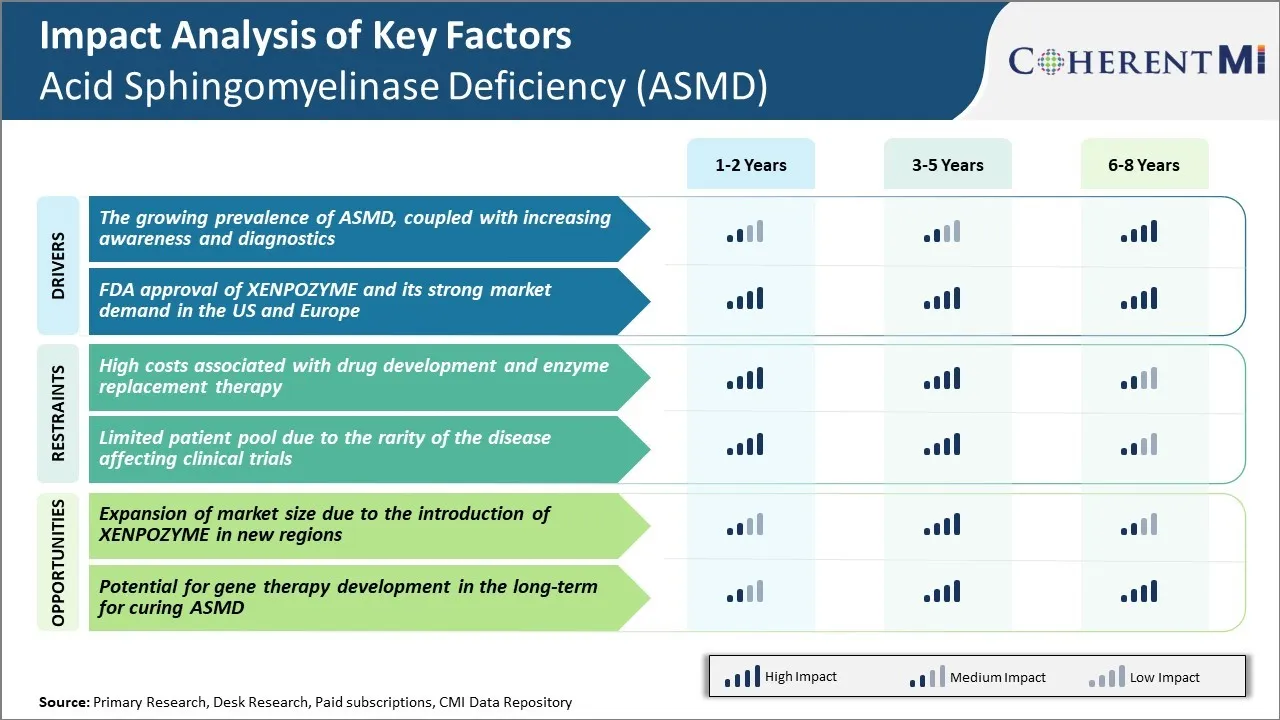
Market Challenge - 의약품 개발 및 효소 교체와 관련된 높은 비용 ·
산성 sphingomyelinase 부족 (ASMD) 시장은 이 희소한 질병을 위한 약 발달과 효소 보충 치료와 관련한 높은 비용 때문에 뜻깊은 도전을 직면합니다. 이러한 드문 상태를 치료하는 약물 개발은 작은 환자 인구의 안전과 효능을 입증하는 광범위한 연구 및 임상 시험이 필요합니다. 이 약 개발 과정은 제약 회사에 대한 매우 비싸고 위험합니다.
또한, Cerdelga라는 승인 된 효소 보충 치료는 연간 $ 300,000 이상의 가격으로 많은 의료 시스템과 환자에게도 비용이 많이 들었습니다. 작은 환자 수는 산성 sphingomyelinase 부족 (ASMD) 시장의 전반적인 크기를 의미하며 제약 회사에 대한 작은 수익 잠재력을 제공하여 약물 개발 투자를 회복시킵니다.
높은 생산 비용은 또한 복잡한 생물학적 성격 때문에 효소 보충 치료에 incurred. 이 중요한 재정적인 도전은 ASMD에 대한 기존 치료법에 대한 새로운 치료 옵션과 환자의 접근 제한으로 더 연구할 수 있습니다.
Market Opportunity - 새로운 지역의 XENPOZYME 소개로 인해 시장 규모 확장
새로운 ASMD 약물 XENPOZYME의 승인 및 상용화는이 조건의 시장 크기를 확장하는 주요 기회를 나타냅니다. XENPOZYME, Xenetic Biosciences에 의해 개발, 임상 시험에서 안전과 효능을 입증했다.
규제 승인으로, 회사는 2023 년 미국의 주요 시장에서 XENPOZYME를 출시 할 계획입니다. 이것은 중요한 추가 처리 선택권을 가진 이 새로운 지구에 있는 ASMD 환자를 제공할 것입니다. XENPOZYME의 소개는 또한 크게 전반적인 산 sphingomyelinase 부족 (ASMD) 시장을 성장하는 잠재력을 가지고 있습니다.
XENPOZYME는 자격이 된 환자 중 uptake를 얻고, 현재 Cerdelga에 의해 개최 된 시장의 일부를 상쇄 할 수 있습니다. 이 시장 확장은이 드문 질병 인구에 대한 치료법을 개발하는 회사에 대한 투자를 개선하는 데 도움이 될 것입니다.