Antibody-mediated 거부 시장 규모 및 점유율 분석 - 성장 추세 및 예측 (2024 - 2031)
Antibody-mediated Rejection Market은 치료 (Plasma Exchange, IVIG, Complement Inhibitors, Proteasome Inhibitors), 개발 (Clazakizumab, Imlifidase)의 약으로, Ther....
Antibody-mediated 거부 시장 트렌드
Market Driver - 글로벌 조직의 수 증가
장기 기부에 대한 인식, 최첨단 외과 기법과 결합, 성공적으로 지금 일반에 복잡한 이식 절차를 수행하기 위해 의사를 활성화. 이것은 크게 매년 전세계에서 수행 된 기관 이식의 총 수를 밀어. 예를 들어, 미국의 Organ Procurement 및 Transplantation Network (OPTN)의 데이터는 지난 10 년 동안 꾸준히 증가한 볼륨이 2021 년에 수행 된 40,000 개 이상의 이식이 있음을 보여줍니다.
그러나, 잘 일치한 기증자에서 오는 기증자 기관에도 불구하고, 수령인의 면역 체계에 의해 항상 거절의 위험이 있습니다. 여기에서는 면역 체계를 억압하는 약의 역할 또는 그것의 거절 기계장치와 방해합니다. 전 세계적으로 상승하는 이식률로, 항환 약을 위한 수요는 탠덤에서 성장합니다.
제약 회사는 더 효과적인 표적 면역 억제제 개발에 크게 투자하고있다. 몇몇 신약 대리인은 또한 면역력을 유도하는 것을 목표로 하고, 결국 이식 환자가 긴 뛰기에 있는 immunosuppression를 얻습니다. 이러한 모든 요인은 잠재적으로 수명을 필요로 할 수있는 환자 풀을 지속적으로 확장하기 위해 포인트를, 따라서 관련 시장을 추진.
Market Driver - Immune System의 Rejection Mechanisms 대상 치료의 발전
의료 과학은 급속한 속도로 발전하고, 이식 면역 분야에서 수많은 유익한 변화를 가져다줍니다. 연구자들은 면역 체계가 비 자기 조직을 인식하고 공격을 시작시키는 방법에 대해 자세히 설명했습니다. 제약 회사는 차세대 prophylactics 개발에 초점을 맞춘 전용 연구 및 개발 노력을 통해 면역성이 높은 통찰력을 활용하고 있습니다.
일부 예로는 면역 세포 또는 항체 중화성 염증성 세포에 특정한 공동 자극 분자를 억제하는 생물학 약물이 포함되어 있습니다. 이 생물학을 평가하는 임상 학문은 넓은 억제 없이 면역 활성화의 효과적인 억제를 보여주고, 개량한 초안 기능 및 환자 생존에 번역. 다른 promising arena는 특정 유전자의 소개 또는 침묵에 의해 유전자 치료는 이식 기관에 항원 별 비 반응성 또는 조작 공차를 생성할 수 있습니다. 이러한 타겟팅 전략은 긴 실행에 기존의 비특성 immunosuppressants를 대체하는 것으로 예상됩니다.
또한, 혼합 칠머리즘 파악을 통해 이식 공차 유도와 같은 기술 약속. 여기, donor에서 뼈 marrow 줄기 세포는 donor와 수신자 사이 상호 hematopoietic 세포 인구를 수립하기 위해 주입됩니다. 이 소설 치료 시작에서 결과의 결과 축적으로, 그들은 anti-rejection 약물 사용 포스트 변환을 변환 할 수있다. 이 백드롭에 대한 전체 시장의 강력한 성장 역동성을 검증합니다.
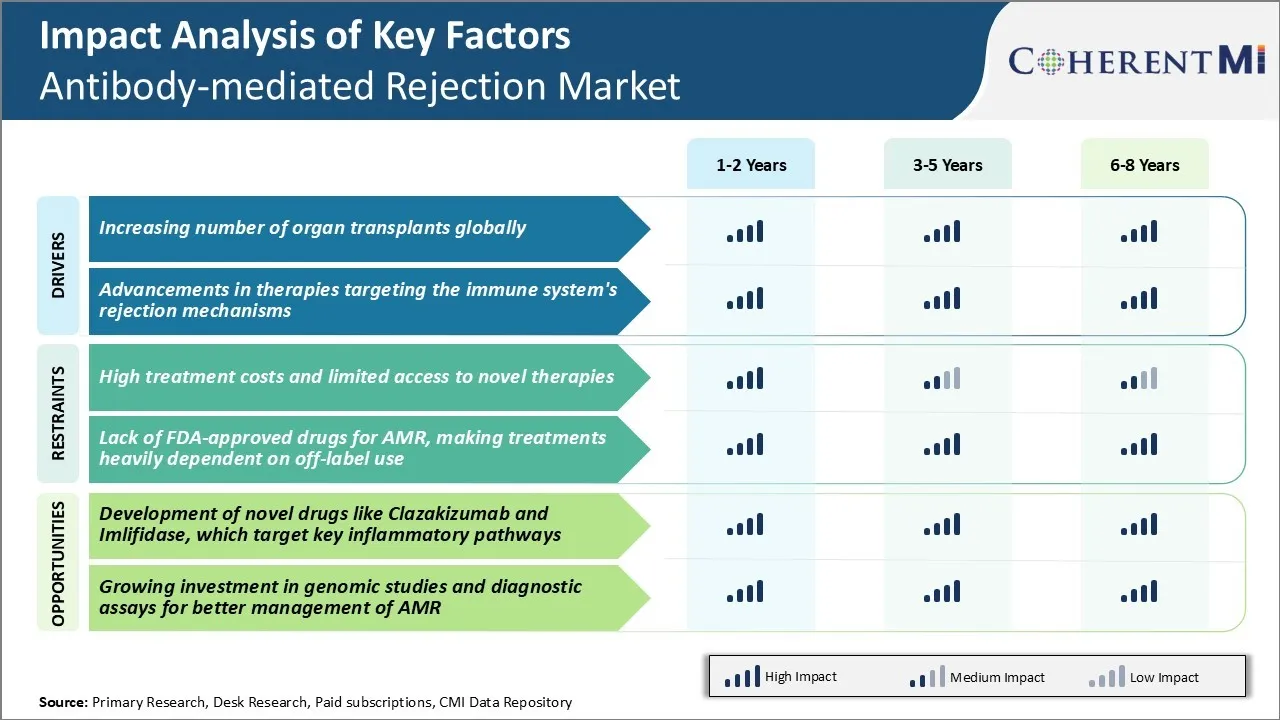
Market Challenge - 새로운 치료에 대한 높은 치료 비용 및 제한된 액세스
항체치료제 시장이 직면한 주요 과제 중 하나는 새로운 치료에 대한 치료와 제한적 접근의 높은 비용입니다. 항체 치료는 infliximab, rituximab 및 정맥 immunoglobulin과 같은 immunosuppressive 약의 사용을 포함합니다, 환자와 의료 시스템에 매우 비쌉니다. 항체 치료를위한 평균 연간 약 비용은 환자 당 $ 20,000에서 $ 100,000까지 범위로 추정되었습니다. 또한, 발달에서 아직도인 많은 신 치료는 또는 최근에 찬성된 찬성되었습니다 널리 접근하거나 다른 요인 때문에 모든 환자를 위해 적당한. 보험 계획 및 건강 시스템에 의해 부분적인 적용은 몇몇 환자를 위한 도달의 이 더 새로운 처리 선택권을 만듭니다. 항체중의 높은 경제적 부담은 환자의 건강 관리 예산의 지속 가능성에 대한 광범위한 접근을 위해 어려움을 포즈합니다. Wider는 소설과 신흥 치료의 채택은 또한 기존 옵션에 우수한 임상 혜택을 민주화하는 데 따라 달라집니다뿐만 아니라 payers와 약물 가격의 성공적인 협상. 이 접근 도전은 전 세계 항체 치료 주사 환자에 대한 치료 결과를 개선하기 위해 해결되어야한다.
Market Opportunity - 목표 키 불임약 개발 오시는 길
항체중환시장의 주요 기회는 상태와 관련된 새로운 약물의 개발에 있습니다. 예를 들어, Clazakizumab은 IL-6에 대한 단일 클론 항체로 임상 시험에서 급성 거부 에피소드를 감소시키기 위해 약속을 보였습니다. IL-6는 이식 기관에 항체 치료 상해에서 중요한 pro-science cytokine implicated입니다.
유사하게, Imlifidase는 항체 약한 거절을 방아쇠에 있는 중앙 역할을 하는 효소 치료입니다. 단계 2 시험에서는, Imlifidase는 신장 이식의 앞에 높게 민감하는 환자를 감축하기 위하여 efficacy를 설명했습니다.
새로운 행동 메커니즘으로, Clazakizumab과 Imlifidase는 기존 치료에 비해 우수한 임상 결과를 제공 할 수 있습니다. 앞으로 몇 년 동안 그들의 승인은 높은 항체 수준을 가진 환자 subgroups에서 unmet 필요를 해결할 수 있었습니다.
또한, 새로운 염증 통로를 타겟팅하는 것은 immunomodulators와 synergistic 조합 치료에 대한 옵션을 산출 할 수 있습니다. 이것은 환자의 임신과 삶의 질 향상에 중요한 기회를 나타냅니다.