Carcinoma Tómico Mercado das Drogas ANÁLISE DE TAMANHO E PARTICIPAÇÃO - TENDÊNCIAS DE CRESCIMENTO E PREVISÕES (2024 - 2031)
Carcinoma Tómico O Mercado de Drogas é segmentado pela Rota da Administração (Oral, Intravenoso, Subcutâneo), Por Molecule Type (Monoclonal Antibody, ....
Carcinoma Tómico Mercado das Drogas Tamanho
Tamanho do mercado em USD Mn
CAGR7.53%
| Período de estudo | 2024 - 2031 |
| Ano base da estimativa | 2023 |
| CAGR | 7.53% |
| Concentração de Mercado | High |
| Principais jogadores | Alphamab, Merck & Co., Inc., Pfizer Inc., GlaxoSmithKline plc, Johnson & Johnson e entre outros |
por favor, avise-nos!
Carcinoma Tómico Mercado das Drogas Análise
Estima-se que o mercado de drogas de carcinoma timático seja avaliado em USD 380 Mn em 2024 e é esperado alcançar USD 631.5 Mn por 2031, crescendo a uma taxa de crescimento anual composta (CAGR) de 7,53% de 2024 a 2031. A pesquisa mostra drogas promissoras no pipeline estão apoiando o crescimento do mercado. Drogas como Tecentriq e Opdivo receberam aprovação da FDA nos últimos anos, melhorando os resultados do tratamento e impulsionando a demanda.
Carcinoma Tómico Mercado das Drogas Tendências
Driver de Mercado - Aumento da demanda por Imunotherapies Inovadoras que visam cânceres raros como o carcinoma timânico
Nos últimos anos, tem havido crescente interesse dentro da comunidade de pesquisa oncológica para desenvolver tratamentos inovadores para tipos de câncer raros e menos comuns, como carcinoma timático. Vários novos estudos surgiram avaliando drogas que podem ajudar a recrutar, ativar e sustentar uma resposta imunológica antitumor especificamente adaptada ao perfil molecular dos tumores de carcinoma timático.
Inibidores de postos de controle direcionados a moléculas como PD-1 e CTLA-4 geraram algum otimismo, pois os resultados iniciais do ensaio clínico demonstraram incentivar as taxas de resposta e a durabilidade da resposta em comparação com a quimioterapia convencional. No entanto, mais pesquisas ainda são necessárias para otimizar essas terapias, dada a heterogeneidade dentro da população paciente e limitações como resistência primária ou adquirida.
Além dos moduladores de postos de controle, há pesquisas substanciais avaliando outras modalidades imunoterapêuticas como o receptor de antígeno quimérico (CAR) Terapia de células T, vacinas contra câncer e terapias de citocina contra carcinoma timático. Os ensaios clínicos precoces caracterizam novos candidatos a drogas que podem melhorar o tráfico imune de células em tumores, estimular as células imunológicas para melhor reconhecer os antígenos do câncer, ou co-estimular as células efetoras imunes uma vez ativado.
No geral, há otimismo dentro da comunidade clínica que, com maior compreensão da imunobiologia subjacente carcinoma timático, e alavancando insights de outros nichos de câncer que foram revolucionados pela imunoterapia.
Driver de mercado - Aumento do investimento em ensaios clínicos para terapias anticorpos monoclonais
Os anticorpos monoclonais surgiram como uma classe importante de drogas cancerosas nos últimos tempos, com vários anticorpos de blockbuster sendo aprovados para uma variedade de cânceres. Sua capacidade de agir através de mecanismos distintos, como bloquear vias de sinalização que conduzem o crescimento do tumor, aumentando as respostas imunológicas contra as células cancerosas ou matando diretamente as células tumorais, as torna valiosas ferramentas terapêuticas.
No caso do carcinoma timic, houve opções de terapia sistêmica padrão-de-cuida limitadas disponíveis até agora além da quimioterapia. No entanto, estudos pré-clínicos promissores identificaram algumas vias de sinalização direcionadas e antígenos tumorais altamente expressos em células de carcinoma timic. Isso levou as empresas farmacêuticas e as biotecnologias a avaliar ativamente vários anticorpos monoclonais em ensaios clínicos contra essa indicação de câncer raro.
Alguns dos estudos em curso estão avaliando anticorpos bloqueando vias conhecidas por serem aberrantemente ativados em carcinoma timático como EGFR, HER2 e VEGF. As empresas estão avaliando os anticorpos monoclonais murinos e humanizados como monoterapias, bem como em combinação com os backbones de quimioterapia. Outras investigações clínicas caracterizam os anticorpos conjugados com cargas citotóxicas com o objetivo de entregar seletivamente agentes de abate de células às células de carcinoma timático. Alguns ensaios de fase inicial também estão explorando conjugados anticorpo-drogas e anticorpos biespecíficos de T-cell para aumentar as respostas imunológicas contra tumores de carcinoma timático.
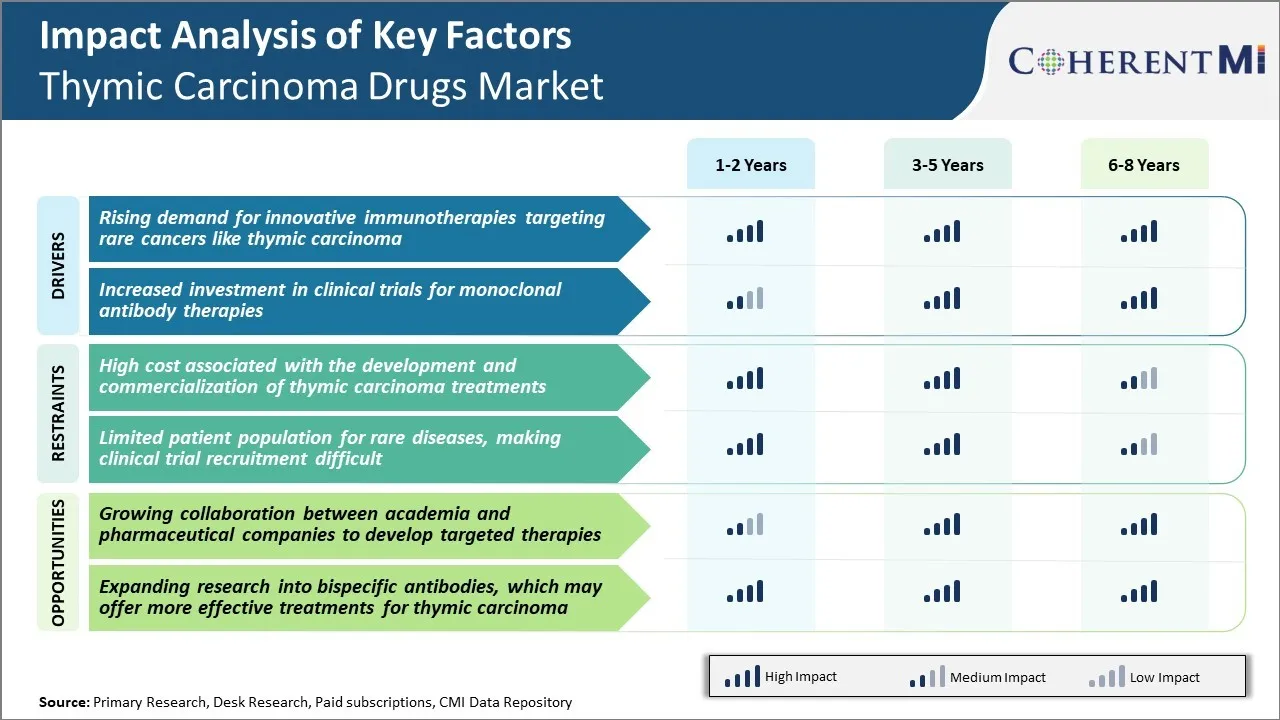
Desafio de Mercado - Alto Custo Associado ao Desenvolvimento e Comercialização de Tratamentos de Carcinoma Tómico
Um dos principais desafios enfrentados pelas empresas que operam no mercado de drogas de carcinomas timáticos é o alto custo associado ao desenvolvimento e comercialização de tratamentos para este raro câncer. Desenvolver novas drogas requer grandes investimentos em pesquisa e ensaios clínicos ao longo de muitos anos. Uma vez que o carcinoma timic tem uma prevalência muito baixa, a associação de pacientes para ensaios clínicos é pequena. Isso torna o recrutamento de julgamento um processo longo e caro.
Além disso, devido aos volumes baixos, as empresas podem achar difícil recuperar custos de desenvolvimento através de vendas de produtos sozinho. Com uma pequena população alvo, os preços das drogas precisam ser muito altos para obter retornos adequados sobre o investimento. No entanto, altos preços de drogas podem reduzir a acessibilidade e acessibilidade para os pacientes.
Em geral, os incentivos comerciais limitados devido ao tamanho do pequeno mercado e aos baixos retornos representam desafios significativos para as empresas farmacêuticas para investir no segmento de carcinomas timic. Isso atua como um dissuasor para novos lançamentos de drogas como as empresas se concentram em áreas terapêuticas mais lucrativas.
Oportunidade de mercado - Colaboração crescente entre academia e empresas farmacêuticas para desenvolver terapias direcionadas
Uma oportunidade potencial no mercado de drogas de carcinoma timático é a crescente colaboração entre institutos de pesquisa acadêmica e empresas farmacêuticas. Com cânceres raros como carcinoma timático apresentando desafios técnicos e comerciais, a parceria entre indústrias é fundamental para o compartilhamento de conhecimento e maximização de recursos.
Os grupos acadêmicos possuem experiência clínica e acesso aos dados dos pacientes, tecidos e amostras biológicas que podem ajudar na compreensão da biologia da doença e na identificação de potenciais alvos de drogas. Enquanto isso, as empresas farmacêuticas fornecem financiamento, conhecimento regulatório e recursos para o desenvolvimento de drogas.
Os últimos anos têm visto um aumento em projetos colaborativos onde pesquisadores acadêmicos e empresas farmacêuticas trabalham em conjunto em várias etapas do processo de desenvolvimento de drogas, desde a identificação do alvo até a validação clínica. Tais alianças visam acelerar a jornada do banco ao lado de novos tratamentos.
Se bem sucedido, eles podem acelerar o lançamento de terapias direcionadas e expandir opções disponíveis para tratar carcinoma timático, beneficiando pacientes e aumentando o crescimento neste mercado.
Preferências dos prescritores de Carcinoma Tómico Mercado das Drogas
O carcinoma tómico é um tumor agressivo que normalmente apresenta em fases tardias. O tratamento de primeira linha envolve quimioterapia, com regimes baseados em Cisplatina ou Carboplatina sendo mais comumente prescritos. Para doença de estágio inicial (I/IIA), pode ser seguido por ressecção cirúrgica, se possível. Em casos mais avançados de estágio IIB/III, as prescrições frequentemente incluem combinações de medicamentos de platina com outros quemotherapeutics, como Paclitaxel, Doxorubicin ou Ifosfamide.
Para pacientes com doença avançada do estágio IV ou aqueles onde a quimioterapia de primeira linha foi mal sucedida, as opções de segunda linha dependem da progressão do tumor e do estado de saúde geral. Os prescritores recomendam frequentemente quimioterapia regente usando drogas como Capecitabine, Gemcitabine ouVinorelbine. Capecitabine (Xeloda) é um fluoropyrimidine oral amplamente utilizado que oferece conveniência em comparação com terapias IV. Para casos de estágio posteriores mais lentos, quimioterapias de baixa dose incluindo drogas orais como Erlotinib (Tarceva) podem ser preferidas.
Outros fatores que influenciam as escolhas do prescritor incluem comorbidades do paciente, sistemas de suporte e linha de tratamentos. Carcinoma tómico tem um prognóstico ruim em geral. Assim, os regimes paliativos enfatizando a qualidade de vida têm prioridade em estágios terminais. Além disso, os prescritores monitoram evidências clínicas emergentes em imunoterapias como inibidores PD-1 que mostram promessa em certos tumores sólidos.
Análise de Opção de Tratamento de Carcinoma Tómico Mercado das Drogas
O tratamento do carcinoma timânico depende do estágio de câncer. Os estágios são determinados pelo tamanho do tumor, espalhados para linfonodos e metástases. Para o estágio inicial I/II doença confinada ao tórax, a cirurgia para remover o tumor (timectomia) oferece a melhor chance de cura. Para estágios mais avançados III/IV, a quimioterapia é geralmente recomendada antes ou depois da cirurgia.
Quimioterapia baseada em platina usando cisplatina ou carboplatina com drogas como doxorubicina é o tratamento padrão de primeira linha. A combinação de cisplatina com doxorubicina mostrou taxas de resposta de 40-60% e oferece uma abordagem de tratamento razoável devido à disponibilidade dessas drogas genéricas. Para pacientes que não são candidatos para platina, o agente simples docetaxel ou paclitaxel são alternativas.
Para carcinoma timico recorrente ou metastático que progride em platina de primeira linha, as opções de segunda linha incluem agentes individuais como vinorelbina, gemcitabina ou drogas orais como capecitabina. Os ensaios clínicos mostraram que a adição de bevacizumab, uma droga anti-angiogênese, à quimioterapia padrão pode melhorar a sobrevivência livre de progressão em pacientes resistentes a platina em comparação com a quimioterapia sozinho. Portanto, a combinação de quimioterapia com bevacizumab tornou-se uma escolha de tratamento de segunda linha preferida onde disponível devido à eficácia melhorada. Medicamentos imunoterapia também estão sob investigação e podem oferecer possibilidades de tratamento futuras.
Principais estratégias vencedoras adotadas pelos principais participantes de Carcinoma Tómico Mercado das Drogas
Foco no desenvolvimento de novas terapias direcionadas: Uma das principais estratégias adotadas pelas empresas tem sido o investimento em pesquisa e desenvolvimento de novas terapias direcionadas para carcinoma timático. Por exemplo, Merck & Co. está desenvolvendo KEYTRUDA (pembrolizumab), uma terapia anti-PD-1, para carcinoma timic. Na fase 2, os testes concluídos em 2018, o KEYTRUDA demonstrou uma taxa de resposta objetiva de 55% em pacientes com carcinoma timic. Este foi um grande sucesso e estabeleceu KEYTRUDA como um potencial novo padrão de cuidado.
Acordos de parceria / licenciamento para o desenvolvimento de drogas: Dada a natureza rara da doença, as empresas individuais não têm piscinas ou recursos suficientes para executar grandes ensaios clínicos sozinho. Parcerias e acordos de licenciamento permitiram que os compostos fossem testados em maiores populações de pacientes. Por exemplo, Daiichi Sankyo fez parceria com a empresa farmacêutica MediGene AG em 2010 para co-desenvolver MT-401, um antagonista do receptor NK-1, para carcinoma timico e outros cânceres.
Foco na região Ásia-Pacífico para ensaios clínicos: Aproximadamente 60% dos novos casos de carcinoma timático ocorrem na Ásia, principalmente no Japão. Reconhecendo esta tendência demográfica, as empresas têm focado esforços significativos de ensaio clínico na Ásia para alcançar uma matrícula mais rápida. Por exemplo, YH25448 da Yuhan Corporation O teste de fase 2 na Coreia e no Japão registrou 46 pacientes com carcinoma timático entre 2016-2018, permitindo uma avaliação eficiente da droga.
Análise Segmental de Carcinoma Tómico Mercado das Drogas
Insights, By Route of Administration: Oral Administration permanece mais preferencial
A rota oral de administração detém a maior parte do mercado de drogas de carcinomas timáticos principalmente devido a conveniência e benefícios de custo para os pacientes. Tomar medicação por via oral permite auto-administração em casa, evitando o consumo de tempo e administração intravenosa mais cara que requer visitas a hospitais ou clínicas. Isso torna o tratamento significativamente mais prático para os pacientes incorporarem em suas vidas diárias.
A conveniência das terapias de carcinoma timático oral significa que os pacientes passam menos tempo recebendo tratamento e mais tempo em outras atividades. Este impacto positivo na qualidade de vida contribui para a alta preferência e demanda por drogas orais. A auto-administração em casa também limita as interrupções no trabalho e compromissos sociais de visitas hospitalares frequentes. O protocolo de baixo custo e administração simples de medicamentos orais faz uso a longo prazo mais sustentável para sistemas de saúde e pacientes.
Os avanços nas tecnologias de entrega e formulação de drogas continuam a aumentar o desenvolvimento de novos tratamentos de anticancer oral. Mais candidatos ao pipeline estão disponíveis ou sendo explorados em forma oral. Esta variedade e o encanamento em expansão sustentam a administração oral como a rota dominante impulsionando a quota de mercado. Suas vantagens inerentes sobre administração intravenosa e subcutânea tornaram a via oral altamente popular entre pacientes e médicos para tratamento de carcinoma timático.

Insights, por tipo de molécula: especificidade de alvo e destaques potenciais curativos Anticorpo monoclonal
Os anticorpos monoclonais que visam moléculas específicas envolvidas no crescimento e progressão de células de carcinoma timático contam para a maior parte do mercado de drogas de carcinoma timático. Essas terapias biológicas altamente direcionadas têm revolucionado o tratamento do câncer nas últimas décadas.
Os anticorpos monoclonais apresentam especificidade requintada, vinculando apenas aos alvos moleculares pretendidos expressos em células tumorais. Esta precisão permite eliminar o câncer sem danos colaterais aos tecidos saudáveis, resultando em menos efeitos colaterais relacionados ao tratamento em comparação com a quimioterapia tradicional. O gerenciamento de efeitos colaterais é uma preocupação crítica do paciente, e o perfil de tolerabilidade favorável dos anticorpos tem impulsionado a demanda pesada.
Algumas terapias anticorpos demonstram potencial para remissão a longo prazo ou cura de carcinoma timático. Ao bloquear vias cruciais de crescimento do tumor, os anticorpos podem restaurar a função normal e interromper a progressão da doença indefinidamente após as extremidades do tratamento. Esta capacidade curativa é um contraste de fome de medicamentos alternativos que só retardam o câncer temporariamente durante a administração. A possibilidade de cura aumenta o interesse do paciente, do médico e do pagador em tratamentos anticorpo.
Os avanços contínuos na biologia molecular e na engenharia anticorpos também contribuem para anticorpos monoclonais que lideram o mercado. À medida que a compreensão melhora das vias aberrantes específicas envolvidas no carcinoma timático, novos anticorpos são desenvolvidos para atingi-los com maior precisão e potência.
Informação adicional de Carcinoma Tómico Mercado das Drogas
- Carcinoma tómico é um câncer raro e agressivo da glândula do timo com uma elevada probabilidade de se espalhar para outros órgãos. Sua gestão requer uma combinação de cirurgia, radiação e terapias de drogas direcionadas, com pesquisas contínuas em imunoterapias que fornecem possíveis avanços.
- O crescente interesse em anticorpos biespecíficos, como o KN046, de Alphamab, representa uma mudança significativa na forma como o carcinoma timic pode ser tratado. Esta nova classe de terapias está mostrando promessa em ensaios clínicos para melhorar os resultados dos pacientes e taxas de sobrevivência.
Visão geral competitiva de Carcinoma Tómico Mercado das Drogas
Os principais jogadores que operam no Mercado de Drogas de Carcinoma Thymic incluem Alphamab, Merck & Co., Inc., Pfizer Inc., GlaxoSmithKline plc., e Johnson & Johnson.
Carcinoma Tómico Mercado das Drogas Líderes
- Alphamab
- Merck & Co., Inc.
- Pfizer Inc.
- GlaxoSmithKline plc
- Johnson & Johnson
Carcinoma Tómico Mercado das Drogas - Rivalidade Competitiva

Carcinoma Tómico Mercado das Drogas
(Dominado por grandes players)
(Altamente competitivo com muitos jogadores.)
Desenvolvimentos recentes em Carcinoma Tómico Mercado das Drogas
- Em julho de 2024, Alphamab está avançando sua droga KN046, um anticorpo biespecífico PD-L1/CTLA-4, em ensaios clínicos de fase III. KN046 visa tumores de expressão PD-L1 elevados e é projetado para melhorar a resposta imunológica, visando o microambiente tumoral. Espera-se que isso ofereça uma nova esperança para pacientes com carcinoma timático.
Carcinoma Tómico Mercado das Drogas Segmentação
- Por via de administração
- Oral
- Intravenoso
- Subcutânea
- Por tipo de molécula
- Monoclonal Anticorpo
- Molécula pequena
- Peptídeo

Gostaria de explorar a opção de comprasecções individuais deste relatório?
Perguntas Frequentes :
Quão grande é o mercado de drogas de carcinoma timático?
Estima-se que o mercado de medicamentos para carcinomas timicos seja avaliado em US$ 380 Mn em 2024 e deverá atingir US$ 631.5 Mn em 2031.
Quais são os principais fatores que dificultam o crescimento do mercado de medicamentos para carcinomas timáticos?
O alto custo associado ao desenvolvimento e comercialização de tratamentos de carcinomas timicos e população de pacientes limitados para doenças raras, dificultando o recrutamento de ensaios clínicos são os principais fatores que dificultam o crescimento do mercado de medicamentos para carcinomas timáticos.
Quais são os principais fatores que impulsionam o crescimento do mercado de drogas de carcinoma timático?
A crescente demanda por imunoterapias inovadoras que visam cânceres raros como carcinoma timático e aumento do investimento em ensaios clínicos para terapias anticorpo monoclonais são os principais fatores que impulsionam o mercado de drogas de carcinoma timático.
Qual é a principal rota de administração no mercado de medicamentos para carcinomas timicos?
A principal rota do segmento de administração é oral.
Quais são os principais jogadores que operam no mercado de medicamentos para carcinomas timicos?
Alphamab, Merck & Co., Inc., Pfizer Inc., GlaxoSmith Kline plc, Johnson & Johnson são os principais jogadores.
Qual será o CAGR do mercado de medicamentos para carcinomas timicos?
O CAGR do mercado de drogas de carcinoma timático é projetado para ser 7.53% de 2024-2031.