临床试验软件市场 规模与份额分析 - 成长趋势与预测 (2024 - 2031)
临床试验软件市场按软件的特性划分(EDC、eCOA/epRO、eConsent),按部署类型划分(On-cloud、On-promises)。 本报告为上述各部分提供了价值(10亿美元)。....
临床试验软件市场 规模
市场规模(美元) Bn
复合年增长率14.3%
| 研究期 | 2024 - 2031 |
| 估计基准年 | 2023 |
| 复合年增长率 | 14.3% |
| 市场集中度 | Medium |
| 主要参与者 | 阿德瓦拉, 亚里士全球, 辅助Rx, 凯利克斯, 克拉里奥 以及其他 |
请告诉我们!
临床试验软件市场 分析
临床试验软件市场估计价值 2024年0.9 Bn美元 预计将达到 (b) 2031年之前为2.3 Bn, 以复合年增长率增长 (CAGR)从2024年到2031年占14.3%. 制药公司对研发的投资增加,对改进数据采集和临床试验管理系统的需求增加,正在推动这一市场的成长。
预计临床试验软件市场在预测期间将稳步增长。 随着技术的进步和提高效率的压力,需要建立临床试验管理系统,以便能够进行基于风险的监测、远程监测技术以及利用远程保健跟踪病人。 这将有助于各组织简化程序,减少与临床试验有关的业务费用。
临床试验软件市场 趋势
市场驱动力 - 加强临床试验的数字化
数字化在保健行业迅速增加,临床试验也不例外。 电子保健记录、远程保健、远程病人监测和其他数字解决方案已经进入主流保健做法,临床试验也出现了同样的趋势。 这一大流行病进一步加快了这一转变,因为旅行限制和社会隔离规范使亲自进行临床试验访问成为挑战,传统的纸面数据收集方法已不再可行。
这促使临床试验利益攸关方采用数字技术和解决办法,以确保审判的连续性。 目前正在利用电子数据采集系统、电子同意、可穿戴设备、应用软件、远程监测装置和远程保健等技术,从现场和病人那里数字采集临床试验数据。 分散式和混合式临床试验模式越来越受欢迎,其中包括分散式和(或)虚拟病人访问。 所有这些变化都增加了对临床试验软件的依赖,为数字临床试验提供动力. 临床试验软件解决方案提供了电子临床结果评估,ePROs,eDiary,数字药品供应管理,远程数据收集等功能,有助于将患者和站点连接起来,并确保数据的无缝采集.
这种日益增长的数字化转变表明,仅靠传统的纸面方法不足以满足目前和未来复杂的临床研究的需求。 数字解决方案带来了提高数据质量和可靠性、使病人能够接触、优化现场表现以及远程纳入不同病人群体的能力等优势。 它们还帮助克服障碍,如数据不准确或不完整、无法采取后续行动、报告延误等,这些障碍困扰着常规试验。 这促使临床试验利益攸关方需要采用专门的软件,以便实现端到端的数字临床试验工作流程,并帮助改变传统试验。 展望未来,数字技术仍将是临床试验设计的核心要素,这些工具与临床试验软件的进一步整合对于今后的试验至关重要。
市场驱动力 - 对以病人为中心的临床试验解决方案的需求增加
临床研究和试验中越来越强调以病人为中心的方法。 患者被认为是重要的伙伴,而不仅仅是主体。 他们的观点、生活质量、舒适和总体的试验经验正得到监管者、赞助者和道德委员会的更多关注。 这种转变既是出于利他主义的考虑,也由于难以征聘和留住病人,使许多审判陷入困境,导致拖延或完全失败。 解决病人的需求,使审判更方便参与者,并让参与者参与,被认为是提高审判可行性的关键。
临床试验软件供应商正在认识到这种转变,并发展更以病人为中心的特征和功能。 这包括易于使用电子同意程序、跨设备的交互式电子PRO和电子日记、可定制的提醒、从家庭监测设备上传任何类型数据的能力、与医生和协调员交谈的选择、状况跟踪、奖项和成就等。 目标是将数据收集工作转变为患者更参与的过程,使其能无缝地适应日常生活。 赞助者还在利用病人社区和数字工具,更好地了解病人的需要,教育病人了解疾病,并提高保健知识。 这有助于提高认识、提高征聘率以及促进参与和遵守规定。
以病人为中心的工具的另一个驱动因素是分散审判。 分散式和混合式模式涉及虚拟和(或)家访的内容,这需要病人在更大程度上具备自我管理能力。 这就需要专门设计临床试验软件,牢记病人的观点,以便他们能够轻松地浏览分散的工作流程。 还正在探索诸如AI、虚拟助理等新兴技术,以便为积极参与分散审判的病人提供个性化信息、提醒和远程援助。
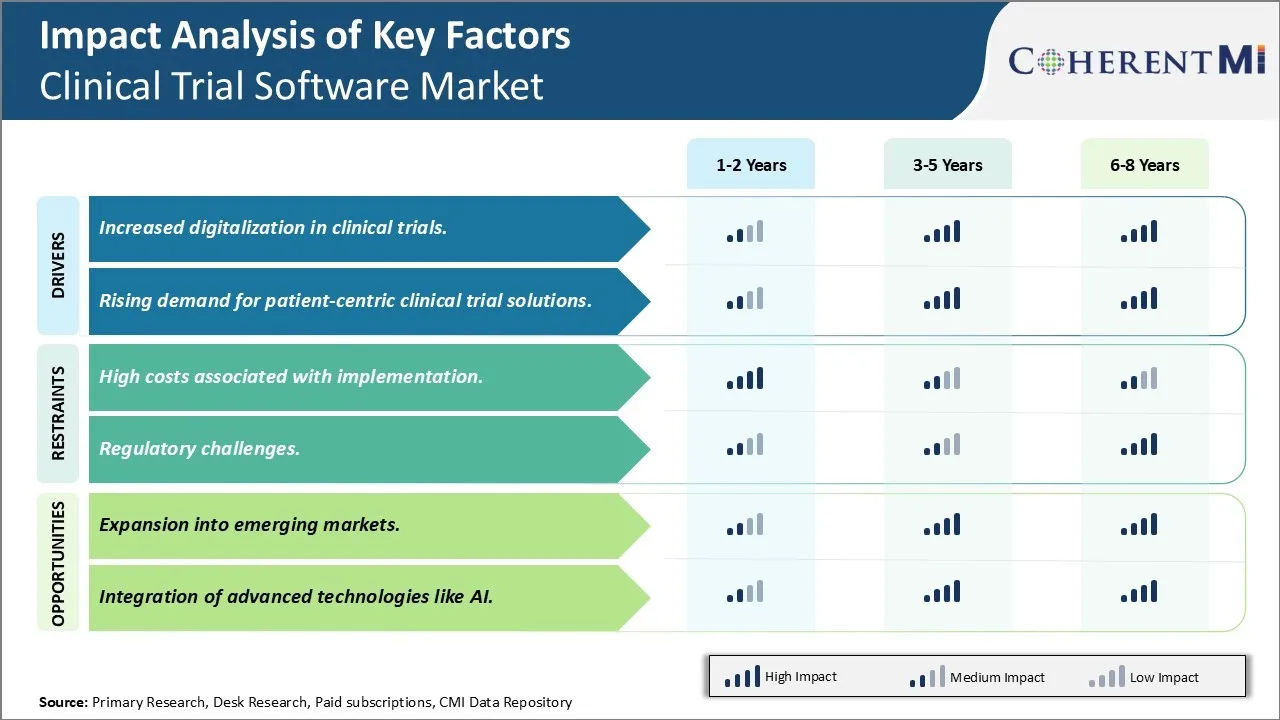
市场挑战----与执行有关的高成本
临床试验软件市场面临的主要挑战之一是实施这些解决方案的成本高昂。 临床试验软件解决方案需要大量的初始投资,而且部署和维护费用很高。 这是因为临床试验软件处理与患者招募,药物分配,数据采集,监管合规等相关的复杂任务. 需要精密的软件工具来有效管理这些复杂的任务。
此外,临床试验软件往往需要根据个别客户的具体要求和临床试验的性质定制。 所有这些因素都导致执行费用高得多。 成本的上升给中小型药材/生物技术公司和临床研究组织带来了压力。 由于预算紧张,许多角色很难证明需要的投资是合理的。 这种高入门障碍限制了先进的临床试验软件的采用,特别是在发展中市场。
市场机会----向新兴市场扩展
临床试验软件市场参与者的主要机会之一是向新兴市场扩展的潜力。 目前,由于药品研发高度集中,临床试验软件市场高度集中在北美和西欧等发达地区. 然而,亚太、拉丁美洲、东欧、中东和非洲等新兴市场预计将带来未来有利可图的增长前景。 保健支出增加、合同研究组织活动增加以及临床试验外包模式的采用增加等因素正在推动新兴区域的需求。
此外,随着监管框架的加强和地方制药业的发展,更多的临床试验正在转向低成本的新兴市场。 这为临床试验软件供应商利用相对未开发的新兴市场提供了重要机会。 努力按照新兴区域客户的要求和预算定制解决方案,对推动采用至关重要。
竞争概览 临床试验软件市场
在临床试验软件市场运营的主要角色包括Advara,Arisglobal,AsidRx,Calyx,Clario,IBM,IQVIA,Medidata,Oracle,Signant Health,和Veeva.
临床试验软件市场 领导者
- 阿德瓦拉
- 亚里士全球
- 辅助Rx
- 凯利克斯
- 克拉里奥
临床试验软件市场 - 竞争对手

临床试验软件市场
(主要参与者主导)
(竞争激烈,参与者众多。)
最新发展 临床试验软件市场
- 2023年8月,德克萨斯州理工大学健康科学中心与深6AI合作,推出了用于临床试验的AI计划.
- 2023年8月,Globant与Medocity合作,在临床研究中加速数字化.
- 2023年7月,ClinOne与PRIMR组建合资公司,以加强临床试验接触.
临床试验软件市场 细分
- 按软件的特性
- 电子数据交换中心
- eCOA/ ePRO
- 同意
- 按部署类型
- 云层
- 内容

您想要了解购买选项吗?本报告的各个部分?
常见问题 :
哪些关键因素阻碍临床试验软件市场的发展?
与实施和管理挑战有关的高昂成本是阻碍临床试验软件市场增长的主要因素。
驱动临床试验软件市场增长的主要因素是什么?
临床试验数字化程度提高,对以病人为中心的临床试验解决方案的需求增加,是推动临床试验软件市场的主要因素.
临床试验软件市场上哪些软件是主要的功能?
软件的主要功能是EDC.
临床试验软件市场的主要操作者是谁?
Advara, Arisglobal, AssidRx, Calyx, Clario, IBM, IQVIA, Medidata, Oracle, Signant Health,和Veeva是主要角色.
临床试验软件市场的CAGR是什么?
临床试验软件市场的CAGR预计从2024-2031年开始为14.3%。