Acute Graft-versus-Host Disease Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Acute Graft-versus-Host Disease Market is segmented By Drug Class (Corticosteroids, Immunosuppressive Agents, Kinase Inhibitors, Mesenchymal Stem Cell....
Acute Graft-versus-Host Disease Market Size
Market Size in USD Bn
CAGR4.10%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 4.10% |
| Market Concentration | High |
| Major Players | MaaT Pharma, Humanigen, Ironwood Pharmaceuticals, Incyte Corporation, Mesoblast and Among Others |
please let us know !
Acute Graft-versus-Host Disease Market Analysis
The Global Acute Graft-versus-Host Disease Market is estimated to be valued at USD 3.9 Bn in 2024 and is expected to reach USD 5.9 Bn by 2031, growing at a compound annual growth rate (CAGR) of 4.10% from 2024 to 2031. The increasing prevalence of cancer and success of hematopoietic cell transplantation procedures are expected to drive the growth of this market.
The market is expected to witness favorable growth over the forecast period owing to rising R&D investments by key players for developing novel treatment options. Advances in understanding the immunological mechanisms of acute GVHD have expanded the pipeline of agents in development which should enhance treatment effectiveness. Monoclonal antibodies targeting cytokines and their receptors are emerging as a promising therapeutic area. The treatment of acute GVHD continues to be a complex challenge owing to the multifactorial pathogenesis underlying this condition. Efforts to develop more targeted and effective therapeutic options that can improve clinical outcomes for patients afflicted by this life-threatening complication post-transplant remain an intense area of focus. In recent years, there has been notable progress in the research and development of novel biologics and monoclonal antibodies that hold promise for more effective intervention in the disease pathways involved in acute GVHD.
Acute Graft-versus-Host Disease Market Trends
Market Driver - Increasing Research and Development of Novel Biotherapeutics and Monoclonal Antibodies Such as Maat013 and Lenzilumab Is Driving Innovation in the Treatment of Acute GVHD.
MaaT Pharma's lead investigational medicinal product, MaaT013, is a microbiome ecosystem therapy that aims to restore a healthy gut microbiome in order to aid in regulating the dysbiotic gut environment often seen in acute GVHD patients. Initial results from Phase 1/2 clinical trials investigating the safety and efficacy of MaaT013 have been encouraging, demonstrating a favorable safety profile as well as signals of clinical activity. The company continues to advance this first-in-class candidate through ongoing trials to further evaluate its potential as a differentiated treatment option for acute GVHD patients. Lenzilumab, a humoral granulocyte colony-stimulating factor neutralizing monoclonal antibody being developed by Humanigen, has also shown early promise in mitigating the incidence and severity of acute GVHD in clinical studies to date. As lenzilumab's mechanism of action targets a key pathway involved in the allogenic response driving acute GVHD, it represents another novel biological approach entering late-stage trials for this patient population.
Continued progress in areas like characterization of immunological pathways, microbiome research, and translational analysis techniques are allowing researchers to gain deeper insights into the complex pathophysiology of acute GVHD. This is enabling the development of novel therapeutic candidates with more selective mechanisms suited to intervene at specific points in the disease process. The results of ongoing clinical research with biologics like MaaT013 and lenzilumab provide early indicators that targeted approaches may offer improved outcomes over conventional options. As such, sustained investment and effort in biotherapeutics and monoclonal antibody research remains an important driver of innovation in the acute GVHD treatment landscape.
Market Driver- Collaboration Between Companies and Academic Institutions
In order to accelerate the development of more effective therapies for acute GVHD, collaboration between industry and academia has become increasingly important. By joining resources and expertise, collaborative partnerships can help address some of the challenges inherent to GVHD research more efficiently. This includes access to specialized patient populations and samples for studies, as well as the consolidated scientific capabilities required for complex projects involving molecular characterization, systems biology analysis and biomarker identification related to this multi-pathway disease.
For instance, MaaT Pharma has established collaborative investigative teams across major transplantation centers in Europe and the US to advance clinical development of its microbiome-based treatments. This provides dedicated investigator sites with experience in acute GVHD, as well as access to patient cohorts needed for evaluation. Beyond any single company, platforms like the Acute GVHD International Consortium bring together leading researchers across disciplines to foster data and sample sharing, harmonize study protocols, develop best practices, and drive forward collaborative projects not feasible for individual groups.
On the industry side, pharmaceutical alliances and licensing deals also help move more candidates into the clinic. For example, humanigen licensed lenzilumab from its original academic developers based on compelling initial data, allowing for progression into late-stage trials. Such partnerships enable greater combined resources and expertise to be leveraged towards the shared goal of developing sorely needed new treatments for acute GVHD patients. Looking ahead, as multi-omic technologies and computational approaches play an expanding role in GVHD research, active collaborations across academic, government and industry stakeholders will remain indispensable.
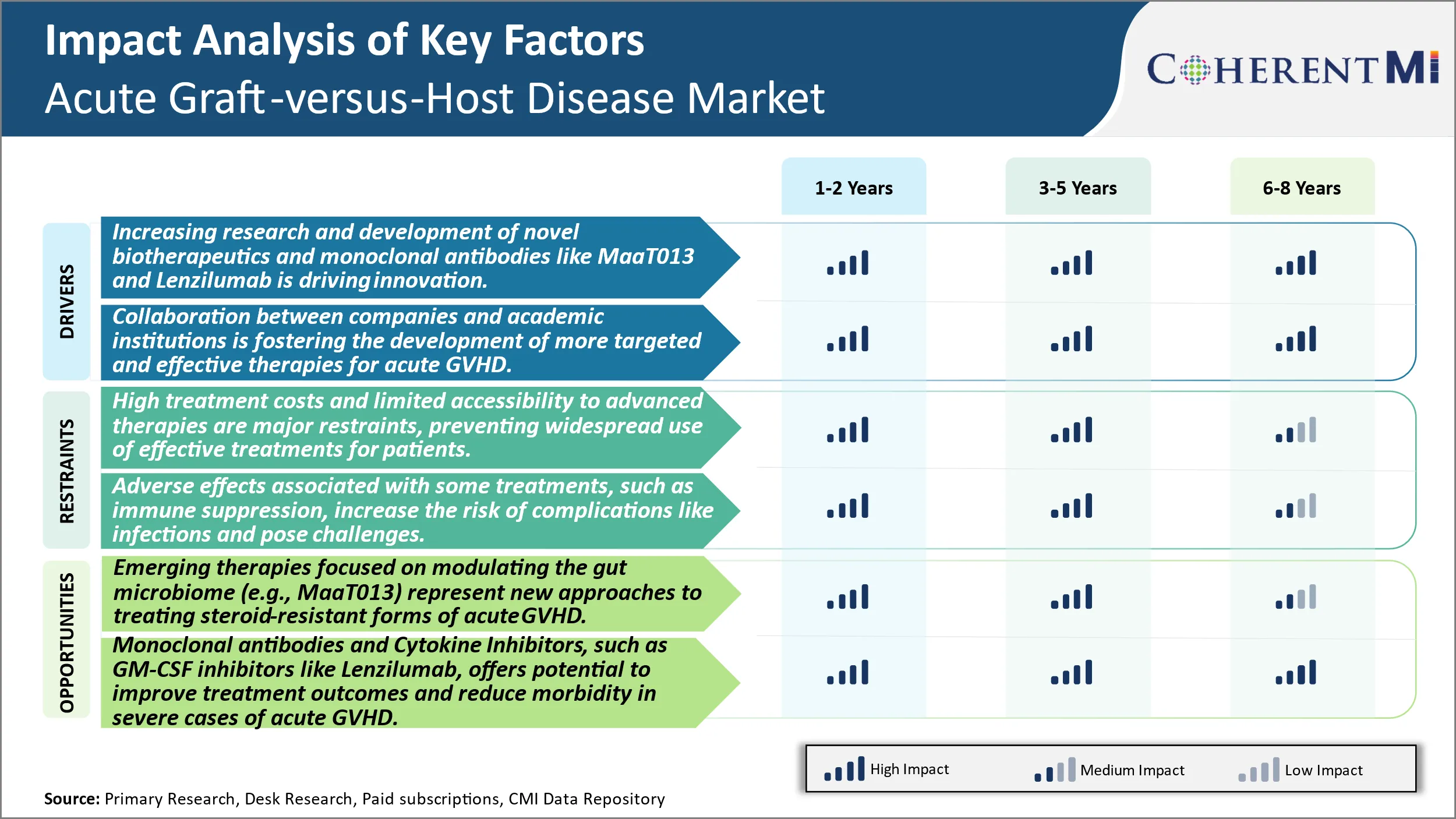
Market Challenge - High Treatment Costs and Limited Accessibility to Advanced Therapies are Major Restraints, Preventing Widespread Use of Effective Treatments for Patients with Severe Acute GVHD.
High treatment costs and limited accessibility to advanced therapies are major restraints, preventing widespread use of effective treatments for patients with severe acute GVHD. The current standard of care for acute GVHD involves the off-label use of corticosteroids as the first-line treatment option. However, corticosteroids are effective in treating only approximately 50–70% of initial acute GVHD cases. For patients who do not respond to steroids or for whom steroids are contraindicated, second-line options such as monoclonal antibodies targeting TNFα, IL-2 receptor, or CD25 have significantly high treatment costs ranging from USD 80,000 to USD 200,000 per course. These elevated costs pose a huge financial burden for patients as acute GVHD treatments can require multiple lines of therapy over extended periods. Furthermore, lack of insurance coverage and limited health resources in underdeveloped regions restrict access to these expensive medications for a large patient population worldwide. Unless novel and affordable treatment alternatives are identified, high costs will continue to impede effective management of steroid-resistant acute GVHD.
Market Opportunity – Emerging Therapies to Boost Gut Microbiome Techniques.
Emerging therapies focused on modulating the gut microbiome (e.g., MaaT013) represent new approaches to treating steroid-resistant forms of acute GVHD, opening doors to targeted interventions. Acute GVHD is known to influence the composition of the gut microbiota in allo-HSCT recipients. Therapies aimed at replenishing depleted commensal bacteria through fecal microbiota transplantation or defined bacterial consortia have demonstrated promising clinical responses. MaaT013, an encapsulated formulation of live biotherapeutic products, has received an orphan drug designation from the FDA for treatment of acute GVHD. In a Phase 2 clinical trial, MaaT013 achieved complete response rates of over 60% and durable responses in steroid-refractory patients with acute GVHD. Such novel mechanisms that work via microbiome modulation are expected to yield superior efficacy and safety profiles compared to standard immunosuppressive drugs. If successful in further trials, gut microbiota-based therapies could introduce more affordable treatment options and improve outcomes for acute GVHD.
Prescribers preferences of Acute Graft-versus-Host Disease Market
Acute Graft Versus Host Disease (aGVHD) is commonly treated via a staged approach based on the severity and organ system involvement. For mild cases affecting only the skin, topical steroids like clobetasol (Temovate) are often prescribed as first-line treatment. However, for more severe or multi-organ cases, systemically-administered corticosteroids remain the standard first-line therapy. Prescribers regularly choose high-dose methylprednisolone (Solu-Medrol) or prednisone to achieve rapid therapeutic effect.
If the patient does not respond to or relapses from first-line corticosteroid therapy, prescribers will typically switch to second-line treatment options. For gastrointestinal or liver aGVHD, mycophenolate mofetil (CellCept) is a commonly prescribed alternative. For cutaneous aGVHD, calcineurin inhibitors like cyclosporine (Gengraf, Neoral) and tacrolimus (Prograf) are frequently used off-label as second-line options.
For steroid-refractory or corticosteroid-dependent aGVHD, prescribers have limited third-line options. Sirolimus (Rapamune), which has a novel mechanism of action, has demonstrated efficacy in clinical trials and is being increasingly prescribed in this setting. Other choices include anti-T-cell therapies like alemtuzumab (Campath) and extracorporeal photopheresis. Newer investigational agents are also gaining interest.
The severity of aGVHD symptoms, risk of complications or relapse, and side effect profiles tend to heavily influence prescribers' line and drug choices within each line of treatment. Close patient monitoring is also paramount.
Treatment Option Analysis of Acute Graft-versus-Host Disease Market
Acute GVHD can be categorized into stages based on severity – mild, moderate, or severe. For mild acute GVHD (stage 1), first-line treatment involves the use of corticosteroids such as prednisone, given at 1-2 mg/kg daily. This immunosuppressive drug helps reduce inflammation and immune reaction. If there is no response within 3-5 days, patients may be given anti-thymocyte globulin (ATG), which contains antibodies against T cells.
For moderate acute GVHD (stage 2), the standard first-line treatment is the combination of prednisone with a calcineurin inhibitor like cyclosporine or tacrolimus. Calcineurin inhibitors work by inhibiting T cell activation. If the condition worsens or does not improve within 2 weeks, second-line options include mycophenolate mofetil (MMF) and monoclonal antibodies like infliximab. MMF selectively inhibits T and B lymphocytes, while infliximab targets tumor necrosis factor-alpha to reduce inflammation.
In cases of severe acute GVHD (stage 3-4), the preferred treatment involves combining methylprednisolone (a stronger corticosteroid) with a calcineurin inhibitor and MMF. For patients who do not respond to steroids, alternative options are sirolimus (an mTOR inhibitor), daclizumab (an anti-CD25 antibody), or ECP (extracorporeal photopheresis). Sirolimus and daclizumab work to suppress T cell responses. This multi-drug regimen along with supportive care aims to reverse the potentially life-threatening effects of severe GVHD.
Key winning strategies adopted by key players of Acute Graft-versus-Host Disease Market
One of the major strategies adopted by companies like Bristol-Myers Squibb and Novartis has been the development of novel drugs that specifically target GVHD. For example, in 2018, Bristol-Myers Squibb gained FDA approval for its drug Inrebic (fedratinib), which is indicated for patients with steroid-refractory acute GVHD. In clinical trials, Inrebic demonstrated a durable complete response rate of 27% and was shown to provide systemic exposure without significant toxic effects. This novel mechanism of action helped make Inrebic a successful treatment option for patients with limited treatment options.
Another strategy adopted by players like Mallinckrodt and Heron Therapeutics has been expanding indications for existing drugs to include acute GVHD. For instance, in 2019 the FDA granted expanded approval for Mallinckrodt's H.P. Acthar Gel to include the treatment of acute GVHD in pediatric and adult patients. Acthar Gel had existing approvals for other conditions, so this expanded label increased the drug's eligible patient population and market share.
Companies are also pursuing M&A activity and partnerships to strengthen their Acute GVHD product pipelines. For example, in 2021 Takeda acquired Lumen Bioscience, gaining access to Lumen's investigational oral biologic therapy for GVHD prevention. This allows Takeda to offer a potential prophylactic treatment which is a major unmet need. Such deals augment existing portfolios in a capital efficient manner.
By developing novel first-in-class drugs, expanding labels of current products, and engaging in strategic deals, these companies have successfully increased treatment options for patients and grown their market shares in the Acute GVHD space through innovative strategies supported by clinical evidence.
Segmental Analysis of Acute Graft-versus-Host Disease Market
Insights, By Drug Class, Corticosteroids Drive Immune Modulation in the Forecast Period.
By drug class, corticosteroids are expected to contribute the highest share 42.3% in 2024 owing to its ability to effectively modulate immune response. Corticosteroids are immunosuppressive agents often used as the first line treatment option due to their ability to inhibit activation and migration of leukocytes to sites of inflammation. They suppress the immune system by activating anti-inflammatory genes, repressing pro-inflammatory genes, and inhibiting production of inflammatory mediators like cytokines and chemokines. This makes them highly effective in mitigating the immune response that causes graft-versus-host disease. Their long-standing use and versatility in modulating immune reactions at various stages has led to corticosteroids becoming the preferred treatment approach initially to control disease symptoms.

Insights, By Distribution Channel, Consolidated Treatment Centers Boosts Significance of Hospitals.
By distribution channel, hospital pharmacies are expected to contribute the highest share 50.2% in 2024 given their role as consolidated treatment centers. Most acute graft-versus-host disease patients receive stem cell transplantation in hospital settings itself. The complex nature of treatments involving aggressive immunosuppression and managing transplant complications requires specialized care that is best provided in hospitals. This makes hospital pharmacies the most direct and convenient source for procuring medications prescribed during transplantation and recovery period. They also ensure consistent supply of drugs needed for inpatient as well as post-discharge care. The steady demand from bone marrow transplant units and availability of a wide portfolio of drugs for related conditions give hospital pharmacies a distinct edge over other distribution channels in this market.
Insights, By Route of Administration, Improved Bioavailability Enhances Oral Route of Administration.
By Route of Administration, Oral administration is expected to contribute the highest share in 2024 owing to improved bioavailability and patient comfort with this route. While intravenous route provides the fastest systemic effect, long-term management relies more on oral medications for their convenience. Recent drug developments have focused on improving oral bioavailability of existing drugs as well as new drug entities. This allows achieving sufficient drug levels in the body with oral administration alone for acute as well as extended treatment phases. Not requiring injections makes oral drugs highly preferable from patient adherence perspective. Additionally, self-administration of tablets/capsules is possible at home post-discharge, avoiding frequent hospital visits. These attributes have revamped the potential of oral drugs for acute graft-versus-host disease.
Additional Insights of Acute Graft-versus-Host Disease Market
Acute Graft Versus Host Disease (GVHD) is a critical complication following allogeneic hematopoietic cell transplantation, with high morbidity and mortality rates. Current treatments focus primarily on systemic steroids as first-line therapy. However, new research highlights innovative approaches, such as microbiome-based biotherapeutics (e.g., MaaT013) and monoclonal antibodies targeting cytokine pathways (e.g., Lenzilumab), which provide promising avenues for patients with steroid-resistant or severe acute GVHD. These developments represent a growing trend towards personalized medicine, targeting the underlying mechanisms of GVHD and improving treatment outcomes. The market for acute GVHD therapies is set to grow as these novel treatments move through clinical trials, with multiple key players, such as MaaT Pharma and Humanigen, leading the charge. The future of acute GVHD treatment looks bright, as new, more effective therapies emerge and collaborations between academia and industry foster innovation.
Competitive overview of Acute Graft-versus-Host Disease Market
The major players operating in the Acute Graft-versus-Host Disease Market include MaaT Pharma, Humanigen, Ironwood Pharmaceuticals, Incyte Corporation, Mesoblast, Merck & Co. Inc, medac GmbH, CSL Limited, Equillium Inc, Cynata Therapeutics Limited, Novartis AG and Bristol-Myers Squibb.
Acute Graft-versus-Host Disease Market Leaders
- MaaT Pharma
- Humanigen
- Ironwood Pharmaceuticals
- Incyte Corporation
- Mesoblast
Acute Graft-versus-Host Disease Market - Competitive Rivalry

Acute Graft-versus-Host Disease Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Acute Graft-versus-Host Disease Market
- In May 2024, MaaT Pharma is advancing MaaT013 in Phase III trials as a microbiome biotherapeutic, aiming to restore the gut microbiome for patients with steroid-resistant acute GVHD.
- In April 2024, Humanigen's Lenzilumab, in Phase II/III trials, aims to neutralize GM-CSF to reduce hyperinflammation in acute GVHD, offering new hope for severe cases.
- In March 2024, Ironwood Pharmaceuticals' Apraglutide, a long-acting GLP-2 analog, is in Phase II trials for treating gastrointestinal symptoms associated with acute GVHD, aiming to improve gut function in affected patients.
Acute Graft-versus-Host Disease Market Segmentation
- By Drug Class
- Corticosteroids
- Immunosuppressive Agents
- Kinase Inhibitors
- Mesenchymal Stem Cells
- Monoclonal Antibody
- By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
- By Route of Administration
- Oral
- Topical
- Intravenous

Would you like to explore the option of buyingindividual sections of this report?
Frequently Asked Questions :
How Big is the Acute Graft-versus-Host Disease Market?
The Global Acute Graft-versus-Host Disease Market is estimated to be valued at USD 3.9 Bn in 2024 and is expected to reach USD 5.9 Bn by 2031.
What will be the CAGR of the Acute Graft-versus-Host Disease Market?
The CAGR of the Acute Graft-versus-Host Disease Market is projected to be 4.10% from 2024 to 2031.
What are the major factors driving the Acute Graft-versus-Host Disease Market growth?
The increasing research and development of novel biotherapeutics and monoclonal antibodies such as Maat013 and Lenzilumab is driving innovation in the treatment of acute GVHD. Collaboration between companies and academic institutions is fostering the development of more targeted and effective therapies for acute GVHD. These are the major factors driving the Acute Graft-versus-Host Disease Market.
What are the key factors hampering the growth of the Acute Graft-versus-Host Disease Market?
The high treatment costs and limited accessibility to advanced therapies are major restraints, preventing widespread use of effective treatments for patients with severe acute GVHD and adverse effects associated with some treatments, such as immune suppression, increase the risk of complications like infections and pose challenges in managing the disease effectively are the major factor hampering the growth of the Acute Graft-versus-Host Disease Market.
Which is the leading Drug Class in the Acute Graft-versus-Host Disease Market?
Corticosteroids is the leading drug class segment.
Which are the major players operating in the Acute Graft-versus-Host Disease Market?
MaaT Pharma, Humanigen, Ironwood Pharmaceuticals, Incyte Corporation, Mesoblast, Merck & Co. Inc, Medac GmbH, CSL Limited, Equillium Inc, Cynata Therapeutics Limited, Novartis AG, Bristol-Myers Squibb are the major players.