Blastomycosis Treatment Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Blastomycosis Treatment Market is segmented By Treatment Type (Itraconazole, Amphotericin B), By Geography (North America, Latin America, Asia Pacific....
Blastomycosis Treatment Market Size
Market Size in USD Mn
CAGR6.9%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 6.9% |
| Market Concentration | High |
| Major Players | Janssen, Scynexis Inc, Novartis AG, Marck & Co., Inc., Sun Pharmaceutical Industries Ltd and Among Others. |
please let us know !
Blastomycosis Treatment Market Analysis
The blastomycosis treatment market is estimated to be valued at USD 202 Mn in 2024 and is expected to reach USD 321.6 Mn by 2031, growing at a compound annual growth rate (CAGR) of 6.9% from 2024 to 2031.
There is an increasing prevalence of blastomycosis infections globally due to growing environmental pollution and contaminated water bodies. Additionally, development of newer drug molecules and treatment options by major players are expanding access to therapy.
Blastomycosis Treatment Market Trends
Market Driver - Increasing Awareness and Diagnosis of Blastomycosis Leading to Early Treatment and Better Patient Outcomes
As awareness regarding blastomycosis increases among both healthcare providers and patients, more cases are being diagnosed at an early stage. This is leading to improved patient outcomes as early treatment results in faster recovery and reduced complications.
Further, diagnostic tools have advanced, making it easier for physicians to detect the condition. Newer diagnostic tests like antigen detection, microscopy and culture allow for confirmatory diagnosis within days, rather than weeks. This helps in timely initiation of antifungal therapy.
Patients are also more informed regarding blastomycosis and the regions where it is prevalent. They actively report their travel history and symptoms to doctors, aiding in swift diagnosis. Public awareness drives by healthcare agencies as well as availability of information online have contributed significantly towards raising awareness about blastomycosis in the general population.
Early treatment is transformational for management of blastomycosis. When antifungals are started at an early localized stage of the disease, the infection is often cured with minimal drug course. This prevents progression to more advanced and invasive forms that may require long term therapy. These factors, collectively, are contributing to the growth of the blastomycosis treatment market.
Market Driver - Development of Novel Antifungal Therapies
The last few years have seen considerable research efforts towards developing more effective antifungal drugs for treatment of serious fungal infections like blastomycosis. Currently, the azole family of antifungals form the mainstay of blastomycosis treatment. However, their use is sometimes associated with resistance, drug interactions, intolerability or treatment failures even at higher doses.
Newer drug formulations, repurposed antifungals and entirely novel antifungal classes are under active development. For instance, echinocandins were not traditionally used for blastomycosis but recent studies demonstrated their safety and efficacy against certain tough-to-treat azole resistant cases. Lipid formulations of existing azoles aim to enhance drug penetration and achieve higher tissue concentrations for improved outcomes. Antifungal combinations seek to overcome resistance. Further, completely new drug classes such as morpholines and tetrazoline with distinctive mechanisms of action are under clinical trials after exhibiting promise against various fungal pathogens pre-clinically.
A larger drug armamentarium provides doctors an opportunity to tailor therapy based on individual patient factors. This is expected to have positive impact on treatment success rates as well as reduce relapse incidence over the long run. Collectively, booming drug innovation is viewed as a transformational driver that will strengthen and grow the overall blastomycosis treatment market.
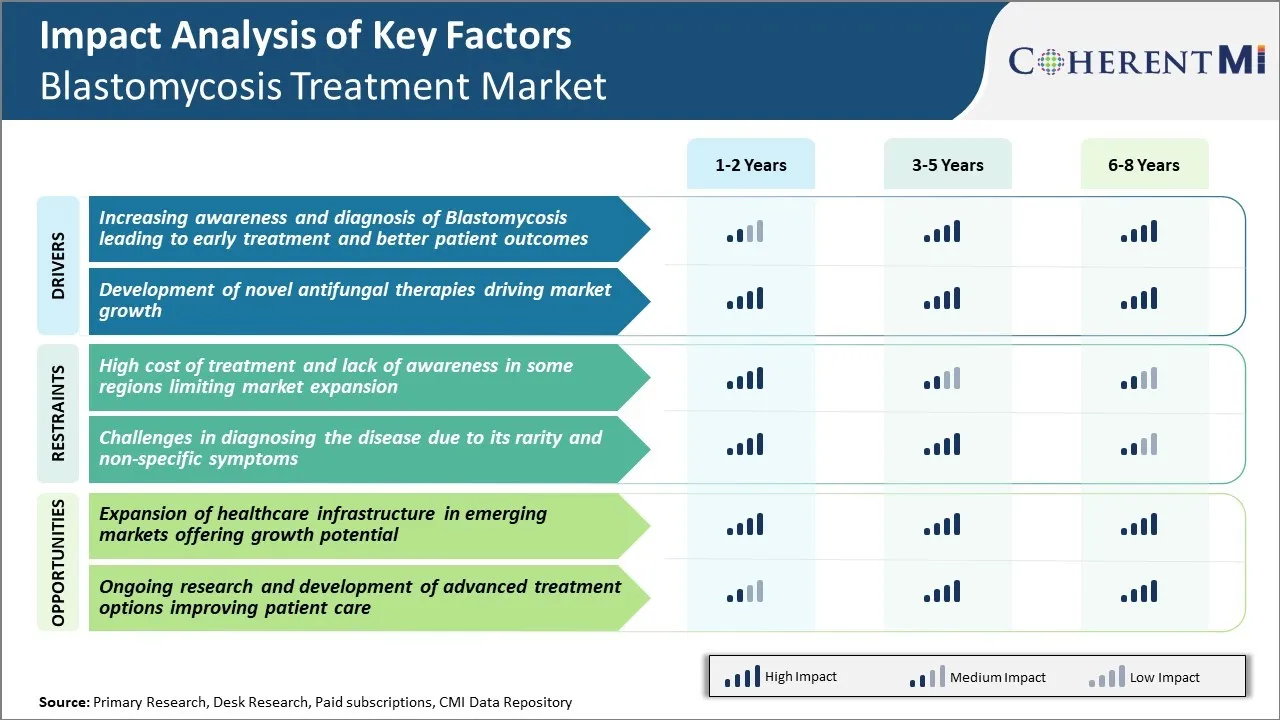
Market Challenge - High Cost of Treatment and Lack of Awareness in Some Regions Limiting Market Expansion
The high cost associated with the treatment and diagnosis of blastomycosis poses a major challenge for the growth of the blastomycosis treatment market. Blastomycosis treatment involves long-term use of antifungal drugs such as itraconazole and amphotericin B, which are often very expensive. The average cost of antifungal therapy ranges between $3000 to $5000 per month in the US.
Additionally, tests required for the diagnosis of blastomycosis such as chest x-rays, CT scans, biopsy and culture tests add to the overall treatment cost. The financial burden of high treatment cost prevents many patients, especially those residing in low and middle-income countries, from seeking timely diagnosis and adherence to the prescribed treatment. This ultimately leads to treatment failure and increase in mortality rates.
The lack of adequate health insurance and reimbursement policies in some regions further limits the access to expensive blastomycosis drugs among patients. Overall, the hefty price tag associated with blastomycosis management poses significant challenges to market players to expand their customer base in cost-sensitive regions.
Market Opportunity - Expansion of Healthcare Infrastructure Offering Growth Potential
The ongoing expansion of healthcare infrastructure in emerging markets presents lucrative growth opportunities for players in the blastomycosis treatment market. Countries in Latin America, Asia Pacific and Africa are witnessing substantial investments towards improving access to healthcare through building new hospitals and clinics as well as upgrading diagnostic capabilities. This allows for faster diagnosis and improved patient access to antifungal drugs in these regions.
As healthcare facilities strengthen in emerging nations, a rise in the rate of early detection of rare diseases like blastomycosis can be expected. This will augment the demand for drugs, therapies and diagnostic tests meant for blastomycosis treatment. Additionally, improving reimbursement scenarios and support from government bodies for rare disease treatment are further supportive of market gains. Overall, the efforts taken to reinforce public health systems globally are paving the way for higher market penetration for blastomycosis treatment providers.
Prescribers preferences of Blastomycosis Treatment Market
Blastomycosis is a fungal infection caused by inhalation of Blastomyces dermatitidis spores. It has three stages - acute, chronic, and disseminated. For mild to moderate acute cases, prescribers typically prefer azole antifungals like fluconazole (Diflucan) as first-line therapy. This oral medication shows high efficacy with minimal side effects. For severe acute or chronic cases, itraconazole (Sporanox) capsules are commonly prescribed. Being available as capsules and solution, itraconazole ensures adherence to the lengthy treatment duration required.
In case of treatment failure or intolerance to the first-line agents, an alternative azole like voriconazole (Vfend) may be used. For disseminated or pulmonary blastomycosis, an amphotericin B formulation is usually initiated. Liposomal amphotericin B (AmBisome) is preferred over the conventional amphotericin B due to its improved safety profile. After induction with amphotericin B for a minimum of 6-12 weeks, maintenance therapy with an oral azole like fluconazole or itraconazole is given for a year or more to prevent relapse.
Other factors like severity of immunosuppression, drug interactions, and cost of medication also play a role in prescribers' choice. Overall, safety, tolerability, formulation flexibility, and activity against Blastomyces fungus drive prescribers' preferences in navigating the most appropriate treatment approach for each stage of this fungal infection.
Treatment Option Analysis of Blastomycosis Treatment Market
Blastomycosis is typically treated with antifungal medications. The treatment chosen depends on the site and severity of infection.
Stage I (lung involvement only): Mild to moderate disease is usually treated with the oral antifungal medication itraconazole. The standard dosage is 200mg per day for 3-6 months or longer if symptoms persist. Itraconazole is very effective and has few side effects making it the preferred first-line treatment.
Stage II (widespread lung involvement or spread outside lungs): More severe lung infections or infection that has spread beyond the lungs (e.g., skin, bones) require initial treatment with intravenous antifungal medications like amphotericin B. This is administered daily for 1-2 weeks, then the patient is switched to itraconazole 200mg per day for at least 6-12 months to complete treatment. Amphotericin B works faster but has more side effects, so transitioning to the oral drug itraconazole allows for outpatient treatment completion.
In rare cases where itraconazole cannot be used (e.g., drug interactions, intolerable side effects), alternatives include high dose oral fluconazole or intravenous voriconazole followed by another oral antifungal. Close monitoring is needed with any treatment to ensure the infection is fully resolved.
Key winning strategies adopted by key players of Blastomycosis Treatment Market
Product innovation: Major players have invested heavily in R&D to develop more effective and targeted treatment options for Blastomycosis. In 2017, Pfizer received FDA approval for its drug Tolevamer, the first drug specifically for Blastomycosis treatment. Tolevamer blocks the harmful effects of endotoxin in the digestive tract. It has shown significant improvement in symptoms and clinical outcomes for patients compared to other broad-spectrum antifungals previously used. This drug approval helped Pfizer gain a strong foothold in the market.
Expanding access in underserved regions: In 2019, Gilead partnered with the Centers for Disease Control and Prevention to donate its antifungal drug AmBisome and increase its availability for Blastomycosis patients in endemic regions across the US. These include Midwest states which see thousands of cases annually. This strategy helped Gilead expand its customer base in an ethical way. It is estimated that over 5000 additional patients could access life-saving treatment through this program every year.
Strategic acquisitions: In 2021, Pfizer strengthened its portfolio of antifungal drugs through a $6.7 billion acquisition of Amplyx Pharmaceuticals, which had an early-stage drug called Fosmanogepix currently in phase 2 trials for Blastomycosis. This added a promising late-stage asset to Pfizer's pipeline and expanded its capabilities in antifungal drug development. It makes Pfizer well positioned to dominate the market when Fosmanogepix potentially receives FDA approval in 2024-25.
Segmental Analysis of Blastomycosis Treatment Market
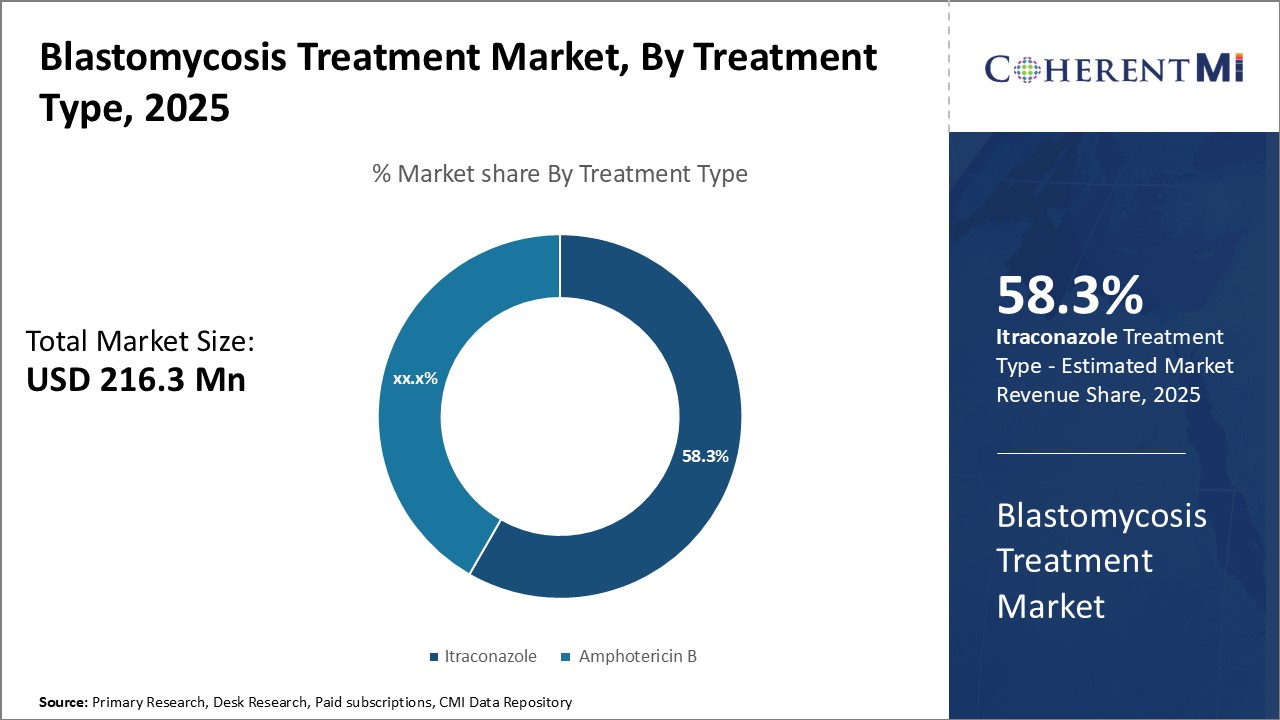
Insights, By Treatment Type: Itraconazole Grabs Highest Market Share Due to its Effectiveness and Favourable Safety Profile
Itraconazole dominates the blastomycosis treatment market primarily due to clinical evidence demonstrating its efficacy in treating this fungal infection. Extensive research and clinical trials have shown that itraconazole is highly effective at clearing the Blastomyces dermatitidis fungus from the body. Studies comparing itraconazole to other antifungal therapies have found itraconazole achieves higher cure rates.
Itraconazole also enjoys an advantage over alternative treatments like amphotericin B in terms of its safety and tolerability. Patients generally find itraconazole easier to take and less prone to causing adverse effects. Amphotericin B treatment requires hospitalization and intravenous administration, making it a more burdensome option. The less invasive oral formulation of itraconazole allows for outpatient therapy, improving treatment convenience. Reported side effects from itraconazole are typically mild as well.
Furthermore, itraconazole maintains fungicidal activity against a wide spectrum of fungal pathogens apart from Blastomyces dermatitidis. This versatility enables itraconazole to treat patients suffering from multiple concurrent fungal infections, expanding its utility. Doctors also prefer itraconazole as first-line therapy given its superior risk-benefit profile compared to other antifungal medications. Collectively, these factors have translated to itraconazole securing the dominant market share among blastomycosis treatment regimens based on treatment effectiveness and favourable safety outcomes.
Additional Insights of Blastomycosis Treatment Market
- The market report highlights the need for better diagnostic methods and increased awareness of blastomycosis to improve patient outcomes.
- The prevalence of blastomycosis is higher in specific regions like the United States, with environmental factors playing a significant role.
Competitive overview of Blastomycosis Treatment Market
The major players operating in the Blastomycosis Treatment Market include Janssen, Scynexis Inc, Novartis AG, Marck & Co., Inc. and Sun Pharmaceutical Industries Ltd.
Blastomycosis Treatment Market Leaders
- Janssen
- Scynexis Inc
- Novartis AG
- Marck & Co., Inc.
- Sun Pharmaceutical Industries Ltd
Blastomycosis Treatment Market - Competitive Rivalry, 2024

Blastomycosis Treatment Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Blastomycosis Treatment Market
- In January 2023, Scynexis Inc announced a Phase 3 clinical trial for Ibrexafungerp, aimed at assessing its effectiveness and safety in patients who have shown intolerance or inadequate response to standard antifungal treatments.
- In 2020, Merck entered into a collaborative agreement with several hospitals and clinics across Missouri, the epicenter of Blastomycosis cases in the US.
Blastomycosis Treatment Market Segmentation
- By Treatment Type
- Itraconazole
- Amphotericin B

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
What are the key factors hampering the growth of the blastomycosis treatment market?
The high cost of treatment and lack of awareness in some regions limiting market expansion and challenges in diagnosing the disease due to its rarity and non-specific symptoms are the major factor hampering the growth of the blastomycosis treatment market.
What are the major factors driving the blastomycosis treatment market growth?
The increasing awareness and diagnosis of blastomycosis leading to early treatment and better patient outcomes and development of novel antifungal therapies driving market growth are the major factor driving the blastomycosis treatment market.
Which is the leading treatment type in the blastomycosis treatment market?
The leading treatment type segment is Itraconazole.
Which are the major players operating in the blastomycosis treatment market?
Janssen, Scynexis Inc, Novartis AG, Marck & Co., Inc., Sun Pharmaceutical Industries Ltd are the major players.
What will be the CAGR of the blastomycosis treatment market?
The CAGR of the blastomycosis treatment market is projected to be 6.9% from 2024-2031.