Hand Foot Syndrome Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Hand Foot Syndrome Market is segmented By Treatment (Systemic Treatments (Oral Medications, Intravenous Therapies), Topical Treatments (Creams, Ointme....
Hand Foot Syndrome Market Size
Market Size in USD Mn
CAGR6.5%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 6.5% |
| Market Concentration | Medium |
| Major Players | Pfizer Inc., Roche Holding AG, Novartis International AG, Merck & Co., Inc., Johnson & Johnson and Among Others. |
please let us know !
Hand Foot Syndrome Market Analysis
The hand foot syndrome market is estimated to be valued at USD 532.5 Mn in 2024 and is expected to reach USD 827.8 Mn by 2031, growing at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2031. With increasing incidence of cancer and approval of new chemotherapeutic drugs, the risk of developing hand foot syndrome as a side effect has also increased. In addition, growing awareness among patients and healthcare practitioners about hand foot syndrome and its available treatment measures are supporting the growth of the hand foot syndrome market.
Hand Foot Syndrome Market Trends
Market Driver - Increasing Usage of Chemotherapy Drugs Causing Higher Incidence Rates of Hand-Foot Syndrome
As cancer being one of the leading causes of mortality worldwide, the need for effective treatment methods has grown exponentially. Chemotherapy has established itself as one of the foremost lines of treatment against cancer, successfully able to extend lives and achieve remission. However, chemotherapy drugs are not without side effects and one such common side effect is hand-foot syndrome.
As per studies, incidence rates of hand-foot syndrome vary based on type of chemotherapy drug used as well as dosage levels but generally fall in the range of 20-40%. For drugs like capecitabine and liposomal doxorubicin, incidences as high as 50-60% have also been reported. The high risk associated with these frequently used drugs means exposure of large patient base to chances of developing hand-foot syndrome.
With global cancer figures projected to continue ascension in coming years owing to aging population and lifestyle changes, chemotherapy treatments shall remain indispensable. This will maintain pressure on drug manufacturers as well as health systems to effectively manage the side effects. As long as chemotherapy usage remains predominant, related adverse effects like hand foot syndrome will persist as a challenge.
Market Driver - Growing Awareness and Demand for Improved Treatment Options
With rising cases of cancer worldwide, greater efforts are being made to enhance awareness among general public as well as medical community regarding the disease and available treatment protocols. This encompasses not just spreading knowledge about the illness itself but also shedding light on common side effects experienced.
Hand-foot syndrome, as one such side effect of selected chemotherapy drugs, has seen increased visibility through various cancer support groups and advocacy platforms. Patients are more informed now about the possibility of developing skin irritations on hands and feet during or after chemotherapy cycles.
Patients look for over-the-counter as well as prescription-based remedies to ease pain, inflammation, swelling and discomfort caused. They also closely follow research on newer drug candidates under investigation. Considering that available treatment choices have shown limitations, the void has created demand for more target-specific and efficacious treatment approaches. Pharmaceutical companies are responding to such needs of patients and healthcare providers by conducting extensive R&D towards development of novel and safe treatment solutions. Support groups chip in as well to support clinical trials through volunteer participation as well as funding support.
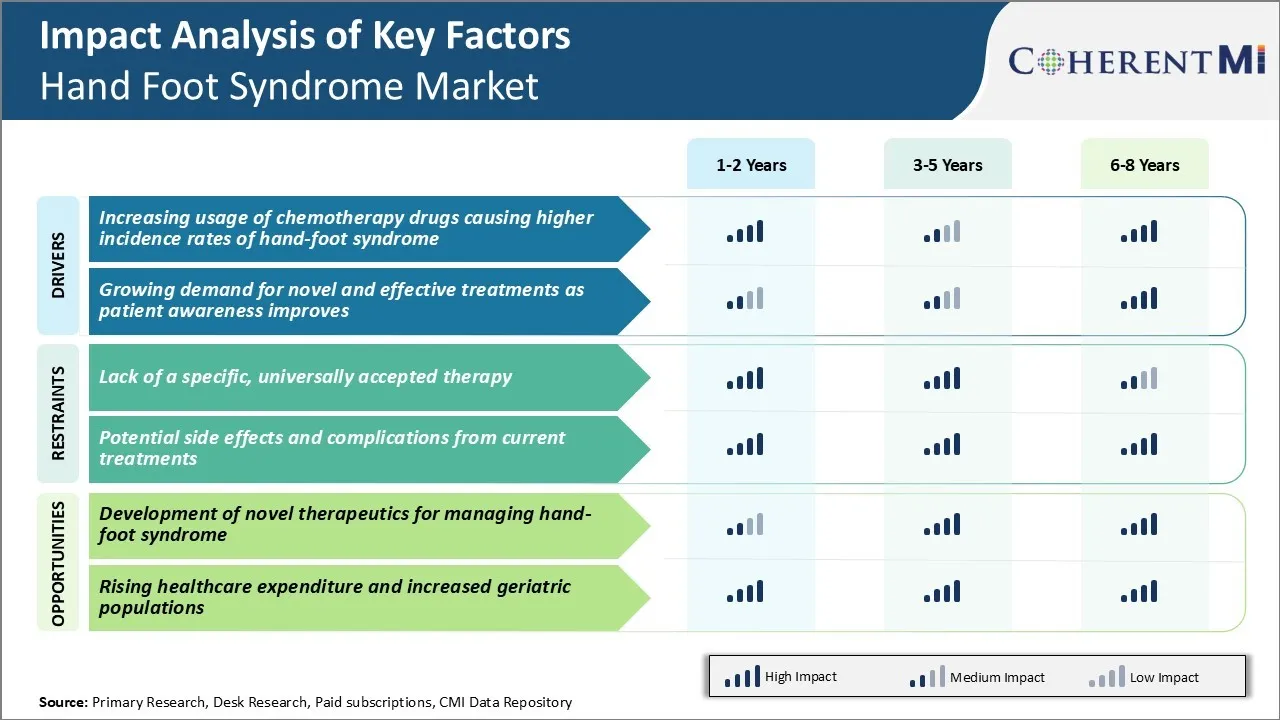
Market Challenge - Lack of a Specific, Universally Accepted Therapy
Hand foot syndrome is a challenging side effect to treat as there remains a lack of a specific, universally accepted therapy for its management. While various treatments have shown some effectiveness, such as topical drugs, supportive care, or dose reductions of the causative cancer drugs, physicians still struggle to prevent its occurrence or adequately treat established cases.
No current options have demonstrated clear superiority over others or provided long lasting relief for patients. This uncertainty surrounding the optimal treatment approach leads to inconsistent care for patients and difficulty designing clinical trials.
Developing new therapeutic strategies is hindered by the lack of biological insight into the root causes of hand foot syndrome. With many cases still resulting in severe pain and blistering, there remains a clear unmet need to identify more reliable and protective therapies to help improve quality of life for the many cancer patients impacted by this debilitating side effect.
Market Opportunity - Development of Novel Therapeutics for Managing Hand-Foot Syndrome
The current lack of effective therapeutic options for hand-foot syndrome presents a major market opportunity for developing novel drug candidates. Successful introduction of new medications that can reliably prevent onset or rapidly resolve symptoms would capture significant market share by addressing this significant unmet medical need.
In particular, targeted, mechanism-based treatments offer the strongest potential to achieve superior efficacy over non-specific supportive care approaches. Proof of meaningful clinical benefit over existing standard of care options could also support premium pricing, reflecting the impact on patient wellbeing and costs of alternative treatments.
Additionally, novel biological insights enabling patient stratification hold promise to elucidate the pathophysiology of vulnerable subsets and their differing response to investigational therapies. Such precision medicine approaches may accelerate clinical development by enriching enrollment for responsive patient populations most likely to benefit.
Overall, the clear lack of existing therapeutic standards indicates a wide-open, hand foot syndrome market for innovative new treatments able to transform management of this debilitating oncology side effect.
Prescribers preferences of Hand Foot Syndrome Market
Hand foot syndrome (HFS), also known as palmar-plantar erythrodysesthesia, is a common side effect of certain chemotherapy drugs. Prescribers typically follow a stepwise approach based on the severity of symptoms.
For mild HFS, over-the-counter topical therapies are prescribed as first-line treatment, such as creams containing urea (Euro-Creme), trolamine (Mycolog II), or lidocaine (Rub A535). If symptoms worsen, topical steroids like clobetasol (Dermovate) may be added.
For moderate HFS, prescription-strength topical steroids are used. Popular options include mometasone (Elocon) and clobetasol (Temovate). Cooling gels containing menthol or camphor (Aspercreme) provide symptomatic relief. Antihistamines like diphenhydramine (Benadryl) may alleviate itching.
In severe cases, dose reduction or temporary discontinuation of the underlying chemotherapy is considered. Wound care involving moisturizers (Aquaphor), silver sulfadiazine cream (Silvadene), and non-adherent dressings (Telfa) is emphasized. Oral pain medications like tramadol (Ultram) or oxycodone (Oxycontin) are occasionally used for pain control.
The stage of the chemotherapy regimen and the patient's overall cancer prognosis strongly influence prescribers' line of treatment for HFS.
Treatment Option Analysis of Hand Foot Syndrome Market
HFS has four stages ranging from mild to severe based on severity of symptoms. Stage 1 or mild HFS involves only pain with no ulceration. Stage 2 involves pain with erythema and swelling without ulceration. Stage 3 has pain with blistering and ulceration less than 2cm, while Stage 4 presents pain with severe ulceration above 2cm.
The preferred treatment line depends on the stage of HFS. For Stage 1, topical therapies like urea or salicylic acid creams are recommended to moisturize skin. Stage 2 responds well to mild corticosteroid creams. If Stage 1/2 treatments fail to provide relief, capecitabine dose reduction is considered.
Stage 3 warrants more potent interventions. Topical calcineurin inhibitors like pimecrolimus or topical corticosteroid combination therapies provide relief in over 60% cases. Alternatively, targeted therapies like palmar-plantar erythrodysesthesia cream containing dyclonine, hydrogenated rosins, and pramoxine hydrochloride help prevent progression to Stage 4.
Stage 4 HFS necessitates hospitalization for intravenous antibiotics and surgical debridement if infection is present. Anti-inflammatory drugs like pentoxifylline may reduce inflammation and promote wound healing. Capecitabine is suspended until recovery. Recent studies indicate that Topical collagen dressing when applied with gentle compression after debridement aids symptom resolution within 2 weeks for Stage 4 HFS.
Key winning strategies adopted by key players of Hand Foot Syndrome Market
Companies have focused on developing novel drug formulations to effectively manage the symptoms of hand foot syndrome. For example, in 2018, Eisai gained FDA approval for Lenvima oral capsules for the treatment of refractory differentiated thyroid cancer. Lenvima demonstrated statistically significant improvement in progression-free survival compared to placebo with a lowered risk of developing hand foot syndrome. This novel formulation helped Eisai gain a competitive advantage in the hand foot syndrome market.
Another strategy adopted is strengthening the pipeline through acquisitions and partnerships. In 2017, Pfizer acquired Anacor Pharmaceuticals, a leader in boron chemistry. Anacor's topical cream Crisaborole (Eucrisa) was approved in 2016 for mild-to-moderate atopic dermatitis. Crisaborole works by inhibiting phosphodiesterase type 4 (PDE-4), reducing inflammation and is well tolerated with no reports of hand foot syndrome. This acquisition strengthened Pfizer's portfolio in dermatology.
Companies also focus on expanding into new therapy areas and obtaining additional approvals for existing drugs. In 2020, Jazz Pharmaceuticals received FDA approval for Rybelsus for chronic weight management. Rybelsus is the first and only glucagon-like peptide-1 (GLP-1) receptor agonist approved in tablet form. The oral formulation significantly lowers the risk of hand foot syndrome compared to injectable drugs. This additional approval expanded Rybelsus' total addressable patient population.
Segmental Analysis of Hand Foot Syndrome Market
Insights, By Treatment: Systemic Treatments Drive Market Share in Treatment Segment
Systemic treatments are estimated to account for 55% share of the hand foot syndrome market in 2024, due to their effectiveness in managing underlying causes of the condition. Many cases of Hand Foot Syndrome are related to chemotherapy used in oncology. Systemic treatments work by addressing the patient's overall health and inhibiting factors that may exacerbate symptoms.
Drugs administered orally or intravenously allow treatment of the root issues like DNA damage and oxidative stress caused by chemotherapy rather than just topical management of dermal symptoms. This makes systemic therapies preferable for oncology patients where controlling the progression of primary diseases is the priority. Oral medications in particular see high demand due to convenience compared to intravascular drugs that require medical supervision.
However, topical treatments still play an important supportive role by providing symptom relief and preventing secondary infections on the skin. Creams and ointments containing agents like urea, aqueous cream, and treatex help moisturize dry areas and form protective barriers. The focus on addressing causal factors pharmacologically through absorption into the bloodstream gives systemic treatments an edge over surface-level options. It positions them as the primary line of defense in serious cases tied to systemic diseases. Their superiority in managing disease progression rather than just symptoms contribute significantly to systemic therapies' pole position in the treatment segment.

Insights, By Application: Oncology Dominates Application Segment Due to Links to Chemotherapy
Oncology is estimated to account for 50% share of the hand foot syndrome market in 2024, in terms of application, mainly because chemotherapy is a leading cause of the condition. A sizeable portion of Hand Foot Syndrome cases stem from cytotoxic agents administered as part of cancer treatment regimens. Chemotherapeutics interfere with DNA synthesis, which can damage skin cells and produce symptoms like redness, swelling, and desquamation.
The prominence of chemotherapy-induced hand foot syndrome within oncology highlights it as a major application field. Patients require options for both preventing and treating dermatological side effects to continue lifesaving cancer therapies unhindered. This presents considerable opportunities for hand foot syndrome products focusing on oncology assistance.
While dermatology and supportive care also contribute applications, the sheer scale of chemotherapy use in fighting cancer means oncology dwarfs other areas. The direct links between chemo agents and hand foot syndrome etiology cement oncology's dominant position. Ensuring cancer patients can complete treatment courses without interruptions from skin issues is a compelling driver of demand within this application segment.
Additional Insights of Hand Foot Syndrome Market
- Prevalence Rates: Approximately 30% of patients undergoing chemotherapy experience Hand-Foot Syndrome, underscoring the need for effective management solutions.
- Regional Growth: Asia-Pacific is expected to witness the highest growth rate in the HFS market due to rising cancer incidence and improving healthcare infrastructure.
- Treatment Adoption: Topical treatments currently hold the largest market share, accounting for 40% of the total market.
- The incidence of hand-foot syndrome is highest among patients receiving VEGFR inhibitors, particularly in the U.S. market. This accounts for about 65% of all cases.
Competitive overview of Hand Foot Syndrome Market
The major players operating in the hand foot syndrome market include Pfizer Inc., Roche Holding AG, Novartis International AG, Merck & Co., Inc., Johnson & Johnson, Quanta Medical, Pfizer, Hoffmann-La Roche, Merck Sharp & Dohme LLC, Ortho Biotech Inc., and Nordic Pharma SAS.
Hand Foot Syndrome Market Leaders
- Pfizer Inc.
- Roche Holding AG
- Novartis International AG
- Merck & Co., Inc.
- Johnson & Johnson
Hand Foot Syndrome Market - Competitive Rivalry, 2024

Hand Foot Syndrome Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Hand Foot Syndrome Market
- In August 2024, Bristol-Myers Squibb launched a new topical treatment to mitigate hand-foot syndrome side effects for patients undergoing VEGFR inhibitors treatment. There is ongoing research and some reports about the use of other topical treatments, such as diclofenac gel, in mitigating hand-foot syndrome caused by chemotherapy agents like capecitabine. These advancements are part of broader efforts by the company to improve the management of this side effect.
- In July 2024, Roche Holding AG entered a strategic partnership with a biotech firm to develop advanced systemic treatments for HFS, focusing on minimizing side effects associated with chemotherapy. Recent significant partnerships involving Roche in 2024 include collaborations with companies like Ascidian Therapeutics to develop gene therapies for neurological diseases and MOMA Therapeutics for oncology treatments focused on dynamic protein targets.
- In March 2024, Novartis announced a partnership with local manufacturers in Europe to expand access to cost-effective hand-foot syndrome treatments. Novartis has also been involved in various collaborations and expansions globally, particularly in pharmaceutical manufacturing and research in other areas, such as radioligand therapy and oncology treatments.
Hand Foot Syndrome Market Segmentation
- By Treatment
- Systemic Treatments
- Oral Medications
- Intravenous Therapies
- Topical Treatments
- Creams
- Ointments
- Systemic Treatments
- By Application
- Oncology
- Chemotherapy-induced HFS
- Other Oncology Treatments
- Dermatology
- Chronic Skin Conditions
- Other Dermatological Uses
- Others
- Alternative Therapies
- Supportive Care
- Oncology

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
How big is the hand foot syndrome market?
The hand foot syndrome market is estimated to be valued at USD 532.5 Mn in 2024 and is expected to reach USD 827.8 Mn by 2031.
What are the key factors hampering the growth of the hand foot syndrome market?
The lack of a specific, universally accepted therapy and potential side effects and complications from current treatments are the major factors hampering the growth of the hand foot syndrome market.
What are the major factors driving the hand foot syndrome market growth?
The increasing usage of chemotherapy drugs causing higher incidence rates of hand-foot syndrome and growing demand for novel and effective treatments as patient awareness improves, are the major factors driving the hand foot syndrome market.
Which is the leading treatment in the hand foot syndrome market?
The leading treatment segment is systemic treatments.
Which are the major players operating in the hand foot syndrome market?
Pfizer Inc., Roche Holding AG, Novartis International AG, Merck & Co., Inc., Johnson & Johnson, Quanta Medical, Pfizer, Hoffmann-La Roche, Merck Sharp & Dohme LLC, Ortho Biotech Inc, and Nordic Pharma SAS are the major players.
What will be the CAGR of the hand foot syndrome market?
The CAGR of the hand foot syndrome market is projected to be 6.5% from 2024-2031.