Medullary Thyroid Cancer Drugs Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Medullary Thyroid Cancer Drugs Market is segemented By Therapeutic Pipeline (Phase I, Phase II, Preclinical), By Route of Administration (Oral, Subcut....
Medullary Thyroid Cancer Drugs Market Size
Market Size in USD Mn
CAGR10.6%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 10.6% |
| Market Concentration | Medium |
| Major Players | Bayer HealthCare, TYK Medicines, Inc, Applied Pharmaceutical Science, Exelixis, Inc., Eli Lilly and Company and Among Others. |
please let us know !
Medullary Thyroid Cancer Drugs Market Analysis
The medullary thyroid cancer drugs market is estimated to be valued at USD 156 Mn in 2024 and is expected to reach USD 315 Mn by 2031, growing at a compound annual growth rate (CAGR) of 10.6% from 2024 to 2031.
The market has been witnessing positive growth trends over the past few years. The increasing incidence of medullary thyroid cancer worldwide and rising demand for targeted therapy drugs are some of the key factors driving the market.
Medullary Thyroid Cancer Drugs Market Trends
Market Driver - Increasing Prevalence of Thyroid Cancers and Rise in Awareness Regarding Early Diagnosis
The medullary thyroid cancer drugs market is witnessing notable growth owing to the rising occurrence of thyroid cancers around the world. The prevalence of thyroid cancer has climbed up substantially in the past few decades, primarily due to growing levels of obesity, changes in reproductive patterns, and other lifestyle factors.
As per expert estimates, the incidence rates of thyroid cancer have nearly tripled in the United States since 1990 and thyroid cancer is now the most common endocrine malignancy. While the exact reasons for this swelling prevalence remain unclear, improved diagnostic methods and heightened screening are also playing a role in detecting cancers earlier.
More importantly, there is a rising social consciousness and understanding among people regarding the importance of early detection of cancers. With enhanced access to information through digital media and a focus on preventive healthcare, many individuals are opting for regular health checkups. This is enabling medical practitioners to detect thyroid tumors at preliminary stages, when treatment yields far better outcomes.
Additionally, voluntary organizations are undertaking various awareness drives and advocacy programs in the community to inform people, especially those at higher risk, about signs and symptoms of thyroid cancer.
Market Driver - Advancements in Targeted Therapies
The medullary thyroid cancer drugs landscape is witnessing noteworthy transformation owing to ongoing transformational research activities. Scientists are tirelessly working on evolving newer targeted treatment approaches with higher efficacy and improved tolerability.
A major area of active research involves targeted therapy drugs that specifically interfere with RET proto-oncogene, a key driver in many cases of medullary thyroid cancer. Several RET inhibitors are in advanced phases of clinical testing and hold promising potential to address the unique oncogenic mutations associated with this cancer subtype.
Additionally, other targeted modalities such as BRAF inhibitors, VEGF/VEGFR inhibitors, immune checkpoint inhibitors and epigenetic modifiers are also under investigation. Research organizations globally are cooperatively conducting extensive biomarker analysis and translational studies to gain deeper insights into molecular pathogenesis of medullary thyroid cancer. Using sophisticated tools such as next-gen sequencing, researchers are working towards developing exquisitely tailored treatments personalized to patients’ unique tumor profiles and clinical phenotypes.
Pharmaceutical firms are making sizeable investments to facilitate such crucial research endeavors through innovative models such as open innovation consortiums and public-private partnerships. These efforts are anticipated to lead to newer treatment paradigms, which could significantly improve medullary thyroid cancer management over the coming years.
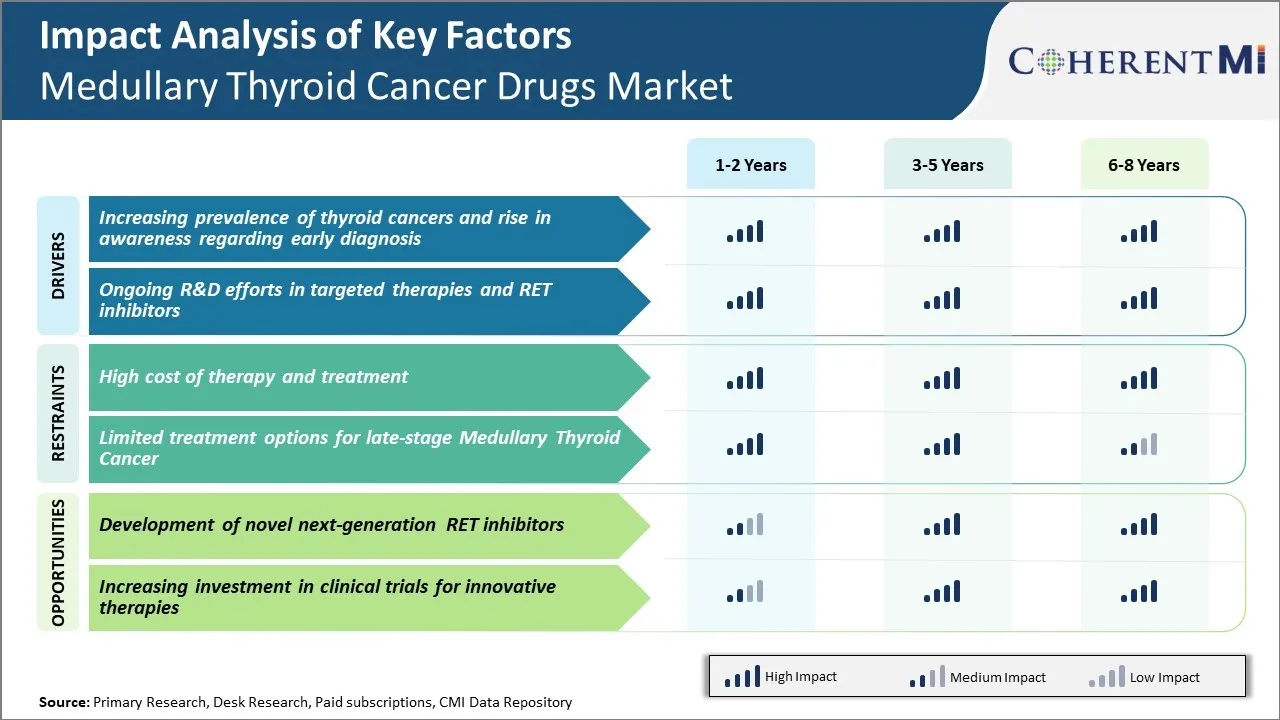
Market Challenge - High Cost of Therapy and Treatment
The high cost of therapy and treatment poses a significant challenge for the growth of the medullary thyroid cancer drugs market. Targeted drug therapies such as vandetanib, cabozantinib and lenvatinib that are used for advanced MTC treatment come with a very high price tag, sometimes exceeding six figures annually for an individual. This not only impacts the affordability for patients but also proves a hurdle in reimbursement negotiations with public and private health insurers.
The socio-economic disparities also influence the access to and uptake of these expensive drugs across different regions and countries. Moreover, the long-term treatment required for MTC patients puts further financial strain on them. Addressing the issues pertaining to the high financial toxicity of drugs will be important for pharmaceutical companies to facilitate greater adoption as well as maintain a stable market position over the coming years.
Market Opportunity - Development of Novel Next-generation RET Inhibitors
The development of novel next-generation RET inhibitors presents a major growth opportunity for players in the medullary thyroid cancer drugs market. Around 60% of MTC cases are caused due to mutations in the RET proto-oncogene which results in uncontrolled cell division.
Current drugs have generally shown effectiveness in treating RET-altered MTC but their overall response rates still remain at around 30-50%. There exists scope for developing newer RET inhibitors that can achieve higher response rates as well as overcome resistance against first-line therapies.
Companies are investing in research exploring selective small molecule RET tyrosine kinase inhibitors and RET-targeted antibody drug conjugates. Some of these pipeline drugs have shown encouraging results in early clinical studies. Their successful approval and commercialization will expand treatment options as well as drive significant revenue for market participants.
Prescribers preferences of Medullary Thyroid Cancer Drugs Market
Medullary thyroid cancer (MTC) treatment varies based on disease stage at diagnosis. For early-stage MTC, total thyroidectomy is the standard first-line treatment. If cancer has spread to local lymph nodes (stage III), prescribers may recommend radioactive iodine ablation drug I-131 (Azedra, Novartis) post-surgery.
For more advanced or metastatic MTC (stage IV), first-line systemic therapies typically involve targeted kinase inhibitors. Cabozantinib (Cabometyx, Exelixis), a multi-kinase inhibitor blocking RET, VEGFR2 and MET receptors, has become a preferred option based on improved progression-free survival shown in pivotal trials. Other RET inhibitors commonly used include vandetanib (Caprelsa, AstraZeneca) and selpercatinib (Retevmo, Eli Lilly).
When disease progresses on first-line options, prescribers may consider other targeted drugs like pralsetinib (Gavreto, Blueprint Medicines) or chemotherapy. For refractory MTC, clinical trials evaluating newer investigational agents like LOXO-292 (Loxo Oncology) may also be considered based on a patient's molecular profile and prognosis.
Key influences on treatment selection include a patient's symptoms, tumor marker levels, prior treatment history, comorbidities and genetic mutations detected. Minimizing treatment side effects while maximizing disease control are high prescribing priorities.
Treatment Option Analysis of Medullary Thyroid Cancer Drugs Market
Medullary thyroid cancer can be divided into four stages - I, II, III and IV - based on tumor size and spread. For stage I/II diseases which are confined to the thyroid, surgery to remove the thyroid (thyroidectomy) is the primary treatment option. For patients with stage III disease where cancer has spread to lymph nodes in the neck, a more extensive surgery (modified neck dissection) along with thyroidectomy is recommended.
For symptomatic or progressive stage IV diseases where cancer has metastasized to other parts of the body, drug therapy is preferred. Two of the most commonly used drug regimens are vandetanib and cabozantinib. Vandetanib (Caprelsa) is a tyrosine kinase inhibitor that works by blocking some of the enzymes involved in tumor growth and spread. It is preferred for symptomatic or progressive medullary thyroid cancer after frontline therapies. Cabozantinib (Cometriq/Cabometyx) another tyrosine kinase inhibitor blocks the RET protein known to drive medullary thyroid cancer.
For palliative care, localization of metastatic lesions by imaging tests and surgical removal or thermal ablation procedures provide relief from symptoms in select cases where it is feasible. External beam radiation therapy may also help control localized bone or brain metastases.
Key winning strategies adopted by key players of Medullary Thyroid Cancer Drugs Market
Focus on targeted drug development: Leading players like Bristol-Myers Squibb, Exelixis and Takeda have focused their R&D efforts on developing targeted drugs to treat MTC. Bristol-Myers Squibb developed Cabometyx (cabozantinib), an oral TKI approved in 2016. It exhibited significant antitumor activity in RET mutation-positive advanced MTC patients. This helped Bristol-Myers Squibb gain a significant market share.
In-licensing/partnership deals: Companies lacking internal expertise have forged partnerships and in-licensing deals. For example, in 2015 Exelixis in-licensed cabozantinib from Bristol-Myers Squibb for certain territories. This reduced Exelixis' development costs and risks. It has since marketed cabozantinib under the brand name Cometriq and seen revenues grow.
Expansion into new indications: Successful drugs are being evaluated in new indications/settings to expand use. Cabometyx is now being studied in adjuvant and neoadjuvant MTC settings which, if successful, could capture more patients earlier in treatment. This strategy of Exelixis helped sustain Cabometyx sales even after losing exclusivity in the U.S.
These strategies of targeted drug development, partnerships and expansion into new settings have helped companies achieve regulatory approvals faster, reduced costs and risks, and maximized commercial potential of drugs like Cabometyx, leading to increased market share and revenues. They have set standards for success in this space.
Segmental Analysis of Medullary Thyroid Cancer Drugs Market
Insights, By Therapeutic Pipeline: Early Innovation Driving Phase I Trials
In terms of therapeutic pipeline, phase I contributes the highest share of the market owing to high investment in innovative drug research. Being the initial testing stage in human clinical trials, phase I sees extensive exploration of new molecular entities for their safety, tolerability and pharmacokinetics.
Given the rarity and limited treatment options for medullary thyroid cancer, pharmaceutical companies are actively pursuing novel targets and mechanisms of action in preclinical research. Drugs entering phase I usually offer new molecular pathways for intervention compared to existing approved therapies. This early-stage evaluation of innovative candidates attracts significant research funding to understand initial human responses.
While phase I has higher investment costs and risks compared to later stages, successful trials lay the foundation for further development. Researchers also gain valuable pharmacokinetic and pharmacodynamic insights to inform future trials. This culture of innovation and willingness to explore early-stage possibilities contributes most to phase I's leading market share within the therapeutic pipeline segment.
Insights, By Route of Administration: Oral Administration Dominating Patient Demand
In terms of route of administration, oral contributes the highest share of the market due to strong patient demand for convenient dosing options. Living with a rare cancer like medullary thyroid entails numerous doctor visits and intravenous infusions that disrupt daily routines.
Patients strongly prefer an oral regimen that can be self-administered at home without medical supervision. This provides greater flexibility, avoids clinic/hospital trips, and has a less visible impact on quality of life compared to subcutaneous injections or intravenous drips. Oral administration is also associated with better treatment adherence when patients can discreetly take pills without disruptive procedures.
Given the chronic nature of medullary thyroid cancer management, oral drugs see high uptake by addressing important patient preferences around dosing convenience and lifestyle impact. This patient demand and receptiveness to oral options drives its significant market share within the route of administration segment.

Insights, By Molecule Type: Monoclonal Antibodies at the Forefront of Targeted Therapies
In terms of molecule type, monoclonal antibody contributes the highest share due to success in exploiting novel targets. Pharmaceutical research has greatly enhanced understanding of the molecular pathways involved in medullary thyroid carcinogenesis.
Multiple drivers and genes associated with disease development and progression have been identified. This expanded molecular knowledge enabled development of targeted monoclonal antibody therapies against dysregulated proteins. Drugs in this class demonstrate high efficacy and specificity by homing in on altered cellular receptors overexpressed in tumors.
Several monoclonal antibodies are in advanced clinical development after achieving promising early results against drivers like RET, NTRK and VEGF. Their novel mechanisms have fewer overlapping toxicities with approved small molecule options as well. This advancement in exploiting specific targets not addressed by existing therapies boosts monoclonal antibody research and uptake within the molecule type segment. Further success stands to solidify this segment's leadership and first-mover advantage over emerging alternatives.
Additional Insights of Medullary Thyroid Cancer Drugs Market
- Various drugs like Regorafenib and TY-1091 are in different phases of clinical development. Regorafenib specifically targets angiogenic and oncogenic pathways critical in Medullary Thyroid Cancer, while TY-1091 is part of a next-generation RET inhibitor platform.
- Medullary Thyroid Cancer constitutes about 3%-4% of all thyroid cancers. Approximately 1,000 new cases are diagnosed each year in the United States. These cancers are rare but often more aggressive than other thyroid cancer types.
Competitive overview of Medullary Thyroid Cancer Drugs Market
The major players operating in the Medullary Thyroid Cancer Drugs Market include Bayer HealthCare, TYK Medicines, Inc, Applied Pharmaceutical Science, Exelixis, Inc., and Eli Lilly and Company.
Medullary Thyroid Cancer Drugs Market Leaders
- Bayer HealthCare
- TYK Medicines, Inc
- Applied Pharmaceutical Science
- Exelixis, Inc.
- Eli Lilly and Company
Medullary Thyroid Cancer Drugs Market - Competitive Rivalry, 2024

Medullary Thyroid Cancer Drugs Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Medullary Thyroid Cancer Drugs Market
- In July 2024, Bayer HealthCare’s drug Regorafenib announced the Phase II clinical trials for the treatment of Medullary Thyroid Cancer (MTC). Regorafenib, a multi-kinase inhibitor, targets various pathways involved in tumor growth, making it a potential therapeutic option for MTC, particularly in cases that are metastatic or refractory to other treatments. This development reflects Bayer's continued efforts to advance treatments that focus on targeted cancer therapies.
- In July 2024, TYK Medicines, Inc. announced that it is advancing its novel RET inhibitor, TY-1091, which is currently in Phase I/II clinical trials. The drug has shown significant anti-tumor activity in preclinical studies, specifically targeting RET mutations. The ongoing trials are focused on evaluating TY-1091 in patients with RET-fusion non-small cell lung cancer, RET-mutation medullary thyroid cancer, and other RET-altered tumors.
- In July 2024, Applied Pharmaceutical Science announced that it is developing APS03118, a promising next-generation selective RET inhibitor. It is currently in the preclinical phase, and has received FDA approval for its Investigational New Drug (IND) application. Additionally, the clinical application for APS03118 has been submitted and approved by the National Medical Products Administration (NMPA).
Medullary Thyroid Cancer Drugs Market Segmentation
- By Therapeutic Pipeline
- Phase I
- Phase II
- Preclinical
- By Route of Administration
- Oral
- Subcutaneous
- Intravenous
- Topical
- By Molecule Type
- Monoclonal antibody
- Small molecule
- Peptide
- Gene Therapy
- By Product Type
- Mono
- Combination
- Mono/Combination

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
How big is the medullary thyroid cancer drugs market?
The medullary thyroid cancer drugs market is estimated to be valued at USD 156 Mn in 2024 and is expected to reach USD 315 Mn by 2031.
What are the key factors hampering the growth of the medullary thyroid cancer drugs market?
The high cost of therapy and treatment and limited treatment options for late-stage medullary thyroid cancer are the major factors hampering the growth of the medullary thyroid cancer drugs market.
What are the major factors driving the medullary thyroid cancer drugs market growth?
The increasing prevalence of thyroid cancers and rise in awareness regarding early diagnosis and ongoing R&D efforts in targeted therapies and ret inhibitors are the major factors driving the medullary thyroid cancer drugs market.
Which is the leading therapeutic pipeline in the medullary thyroid cancer drugs market?
The leading therapeutic pipeline segment is phase I.
Which are the major players operating in the medullary thyroid cancer drugs market?
Bayer HealthCare, TYK Medicines, Inc, Applied Pharmaceutical Science, Exelixis, Inc., and Eli Lilly and Company are the major players.
What will be the CAGR of the medullary thyroid cancer drugs market?
The CAGR of the medullary thyroid cancer drugs market is projected to be 10.6% from 2024-2031.