Merkel Cell Carcinoma Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Merkel Cell Carcinoma Market is Segmented By Treatment Type (Surgery, Radiation Therapy, Chemotherapy, Targeted Therapy, Immunotherapy), By Diagnosis ....
Merkel Cell Carcinoma Market Size
Market Size in USD Bn
CAGR4.1%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 4.1% |
| Market Concentration | High |
| Major Players | Merck, Incyte Corporation, Kartos Therapeutics, Inc., Bristol-Myers Squibb, Amgen Inc. and Among Others. |
please let us know !
Merkel Cell Carcinoma Market Analysis
The Global Merkel Cell Carcinoma Market is estimated to be valued at USD 2.51 Bn in 2024 and is expected to reach USD 3.38 Bn by 2031, growing at a compound annual growth rate (CAGR) of 4.1% from 2024 to 2031. Merkel cell carcinoma is a rare form of skin cancer that is believed to be caused by the Merkel cell polyomavirus. Increasing awareness regarding early diagnosis and treatment of skin cancer is expected to drive the market during the forecast period.
The market is anticipated to experience steady growth owing to factors such as rising healthcare spending across developed countries, increasing R&D investments for development of novel therapies, and growing geriatric population who are prone to developing the condition. Furthermore, improving reimbursement policies and introduction of combination therapies have opened new avenues for market participants in the global Merkel cell carcinoma market.
Merkel Cell Carcinoma Market Trends
Market Driver - Increasing incidence of Merkel Cell Carcinoma due to aging population and UV exposure.
As the global population continues to age, the incidence of Merkel cell carcinoma is rising at an alarming rate. Merkel cell carcinoma is a rare form of skin cancer that typically affects older adults aged 65 years and above. As lifespan increases across developed nations, the proportion of elderly in the overall population is growing steadily. People aged 65 years or older are more susceptible to Merkel cell carcinoma due to age-related weakening of the immune system and deteriorating ability to repair skin damage. At the same time, the widespread use of tanning beds and recreational exposure to UV rays from the sun without adequate protection have been major drivers of rising skin cancer rates. Even occasional exposure to intense UV rays can potentially trigger Merkel cell carcinoma, and such sporadic exposures have become common in recent decades due to changing lifestyles and outdoor recreational activities.
The interaction of growing elderly population sizes with high UV ray exposures from the sun and tanning beds is creating a catastrophic rise in Merkel cell carcinoma cases globally. Countries with large aging populations and sunny climates like USA, Australia, Spain and Italy have witnessed some of the sharpest increases. For instance, incidence rates in Australia have tripled over the past two decades due to high UV index levels year-round coupled with over 23% of the population being aged above 65 currently. Similarly, Florida in USA has emerged as a hotspot due to abundant sunshine and prevalence of retirement communities. Unless awareness rises regarding sun protection for elderly individuals regularly engaging in outdoor activities, this dangerous interaction is projected to continue fueling the global Merkel cell carcinoma disease burden in the coming decades.
Advancements in Immunotherapy Treatments Improving Patient Outcomes
There have been major breakthroughs in immunotherapy for cancer treatment in recent years including for Merkel cell carcinoma. Immunotherapy activates the body's natural defense mechanisms to recognize and attack cancer cells by augmenting the immune response. Two types of immunotherapies namely PD-1/PD-L1 inhibitors and CTLA-4 inhibitors have shown very promising results against Merkel cell carcinoma which was previously difficult to treat. Pembrolizumab and avelumab are PD-1 inhibitors that have been approved by regulatory authorities based on results from late phase clinical trials demonstrating significantly higher response rates and survival benefits compared to chemotherapy - the earlier standard of care. Ipilimumab is a CTLA-4 inhibitor also demonstrating encouraging effectiveness both as a single agent and in combination with PD-1 inhibitors.
These represent a paradigm shift away from non-specific chemotherapy to more targeted treatments leveraging the immune system's precision. Immunotherapies have considerably lower side effect profiles allowing for superior tolerability especially important for an elderly patient population. Nanotherapeutics and oncolytic virus therapies are newer approaches under investigation which could potentially enhance the stimulation of anti-tumor immune responses even more with minimal toxicity. Overall, the expanding armamentarium of novel immunotherapies continues to reshape long-term outcomes for Merkel cell carcinoma positively. Wider patient access to such advances worldwide would aid in optimally managing this historically difficult to treat disease.
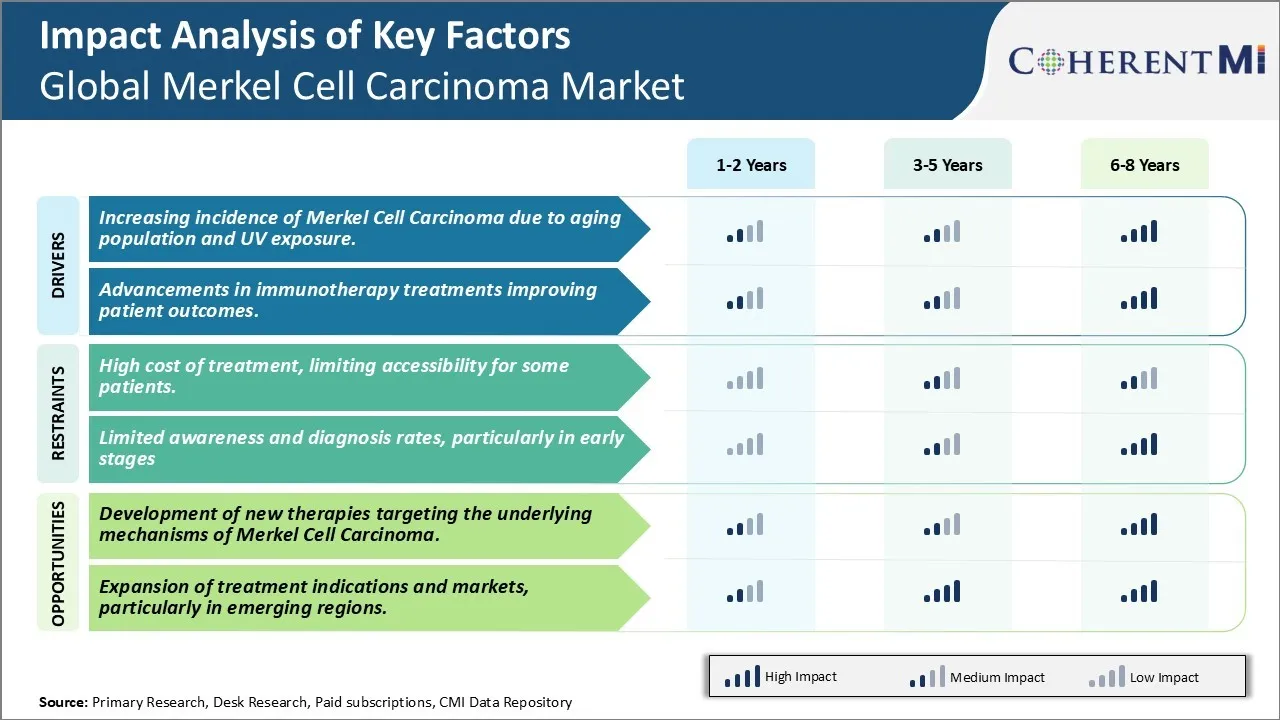
Market Challenge - High cost of treatment, limiting accessibility for some patients.
The high cost of treatment for Merkel cell carcinoma poses a significant challenge for widespread accessibility of care. Currently available therapies such as chemotherapy, radiation therapy and immunotherapy have considerably high price tags, making them unaffordable for some sections of patients. Patients usually require expensive combinations of various therapies such as surgery along with chemotherapy and immunotherapy. This further drives up the total cost of treatment. The added costs of subsequent treatments for recurrence of the condition also burden the healthcare expenditure of patients. Financial toxicity is a major issue, as out-of-pocket spending on MCC treatment pushes some patients into debt or under-insurance. The high eligibility criteria for accessing government insurance or funding likewise leaves many patients without adequate coverage for the long-term costs. Achieving equitable access to existing and emerging therapies remains a critical challenge for maximizing outcomes and quality of life for all MCC patients.
Market Opportunity- Development of new therapies targeting the underlying mechanisms of Merkel Cell Carcinoma.
Promising opportunities lie ahead with ongoing research towards development of new targeted therapies for Merkel cell carcinoma. There is growing understanding of the molecular underpinnings and driver mutations involved in MCC pathogenesis. Recent discoveries have implicated the Merkel cell polyomavirus, as well as alterations in certain cancer pathways such as the MAPK and PI3K-AKT cascades, to play key roles in tumour development and progression. These insights are propelling the design and testing of novel treatments directed at virus-specific and genetically defined subsets of patients. Some clinical successes have been observed with immunotherapies as well as small molecule inhibitors of cancer signalling. Further exploration of mechanisms like viral oncogenesis and immunotherapy response markers could yield more precise targeted agents in the future. Development of affordable combination regimens may also help maximize therapeutic benefit while keeping costs down. With continued advances, new treatment options may significantly improve clinical outcomes and quality of life for Merkel cell carcinoma patients worldwide.
Prescribers preferences of Merkel Cell Carcinoma Market
Merkel Cell Carcinoma (MCC) is typically treated with surgery as the primary treatment for localized disease. For patients presenting with stage I-II MCC, surgical excision with adequate margins is the standard first-line treatment. Chemotherapy may be recommended post-surgery in cases where risk of recurrence is high.
For patients with regional or metastatic MCC (stages III-IV), the first line of treatment typically involves a platinum-based chemotherapy regimen. Cisplatin or carboplatin combined with etoposide is commonly prescribed. Key brand name treatments include Paraplatin for carboplatin and Toposar for etoposide. Responding patients may then undergo surgery to remove any remaining tumors.
For recurrent, persistent or metastatic MCC after first-line chemotherapy, avelumab (Bavencio) has emerged as a preferred second-line treatment option. It is a PD-L1 inhibitor approved by the FDA based on high response rates seen in clinical trials. Prescribers prefer avelumab due to its relatively mild side effect profile compared to conventional chemotherapy. Pembrolizumab (Keytruda) and cemiplimab (Libtayo) are also used as subsequent lines of immunotherapy depending on tumor PD-L1 expression levels.
Other factors influencing treatment choices include age and functional status of the patient, cancer progression timeline and mutations detected through genomic testing. Physicians aim to balance maximizing survival benefits with maintaining quality of life.
Treatment Option Analysis of Merkel Cell Carcinoma Market
Merkel cell carcinoma (MCC) is typically staged using the American Joint Committee on Cancer (AJCC) TNM staging system. Treatment depends on the stage of disease.
For local and locally advanced disease (Stage I-III), surgery is the primary treatment with the goal of wide local excision with tumor-free margins. For those unable to undergo surgery, radiation therapy is recommended. For higher risk resected tumors or those with adverse features, adjuvant therapy with immunotherapy or chemotherapy may be considered.
The preferred immunotherapy drug is avelumab (Bavencio). It is a PD-L1 inhibitor that helps the immune system attack cancer cells. Studies have shown it can improve outcomes when given after surgery in Stage I-III. Pembrolizumab (Keytruda) is also used off-label for adjuvant treatment.
For metastatic MCC (Stage IV), systemic therapy is used. Chemotherapy regimens like cisplatin/etoposide remain standard first-line options. However, immunotherapy drugs like avelumab and pembrolizumab are now preferred due to improved efficacy and fewer side effects compared to chemotherapy. They work by blocking immune checkpoint proteins, enabling the immune system to better target cancer cells. Both drugs have approval for treatment-naive Stage IV MCC based on phase 2 clinical trials showing impressive response rates and overall survival benefits compared to chemotherapy.
Key winning strategies adopted by key players of Merkel Cell Carcinoma Market
Merkel cell carcinoma is a rare and aggressive form of skin cancer that requires effective treatment strategies for improved patient outcomes. Key players in the market have adopted innovative drug development and acquisition strategies to strengthen their product pipelines.
For instance, Merck & Co. acquired Privately held ArQule Inc., a clinical-stage biotechnology company in 2020 for $2.7 billion. This helped Merck gain full rights to ARQ 531, an orally bioavailable, potent and selective inhibitor of both wild type and C481S-mutant BTK, which is being evaluated for hematologic cancers. ARQ 531 has potential as a treatment for Merkel cell carcinoma and this strategic acquisition allows Merck to accelerate its development.
Another example is Amgen acquiring global rights to tepotinib in 2017 through a licensing agreement with Merck KGaA, Darmstadt, Germany and EMD Serono. Tepotinib is an oral MET inhibitor that showed promising clinical activity in patients with Merkel cell carcinoma with MET gene alterations. This strategic in-licensing of a late-stage asset strengthened Amgen's oncology pipeline with a potential new treatment option.
Statistics show that tepotinib led to an objective response rate of 50% in a single-arm Phase II study involving metastatic Merkel cell carcinoma patients with MET gene alterations. This success helped Amgen gain FDA approval for tepotinib in 2021, giving them an early mover advantage in this niche market segment.
These examples illustrate how strategic acquisitions and in-licensing deals have helped key players expand their product pipelines and capture potential blockbuster drugs to treat Merkel cell carcinoma. Such dealmaking strategies have been crucial winning factors as they enable faster development timelines and market access for much needed novel therapies.
Segmental Analysis of Merkel Cell Carcinoma Market
Insights, By Treatment Type- Surgery Leads Treatment Due to Higher Success Rate
In terms of By Treatment Type, Surgery contributes the highest share of the market with 35.2% in 2024 owning to its higher success rate compared to other treatments. Surgery is usually the first line of treatment for Merkel cell carcinoma as it offers the best chance of completely removing the cancer from the body. The goal of surgery is to remove the entire tumor along with some surrounding healthy tissue to prevent leftover cancer cells from regrowing as recurrent disease. The type of surgery performed depends on the size and location of the tumor. For localized tumors, physicians may perform a wide local excision to remove the cancerous tissue along with a border of normal skin. For advanced tumors, lymph nodes in the affected region may also be surgically removed in a procedure called lymph node dissection. Surgery provides the dual benefits of both diagnosis as well as treatment at the initial stages of Merkel cell carcinoma. Its widespread adoption can be attributed to the strong evidence that shows surgical removal of tumors less than 2cm in size leads to 5-year survival rates of over 90%. Even for larger and more invasive tumors, surgery combined with adjuvant therapies leads to significantly better outcomes compared to non-surgical approaches alone. These factors contribute to surgery being the treatment of choice for Merkel cell carcinoma patients worldwide.
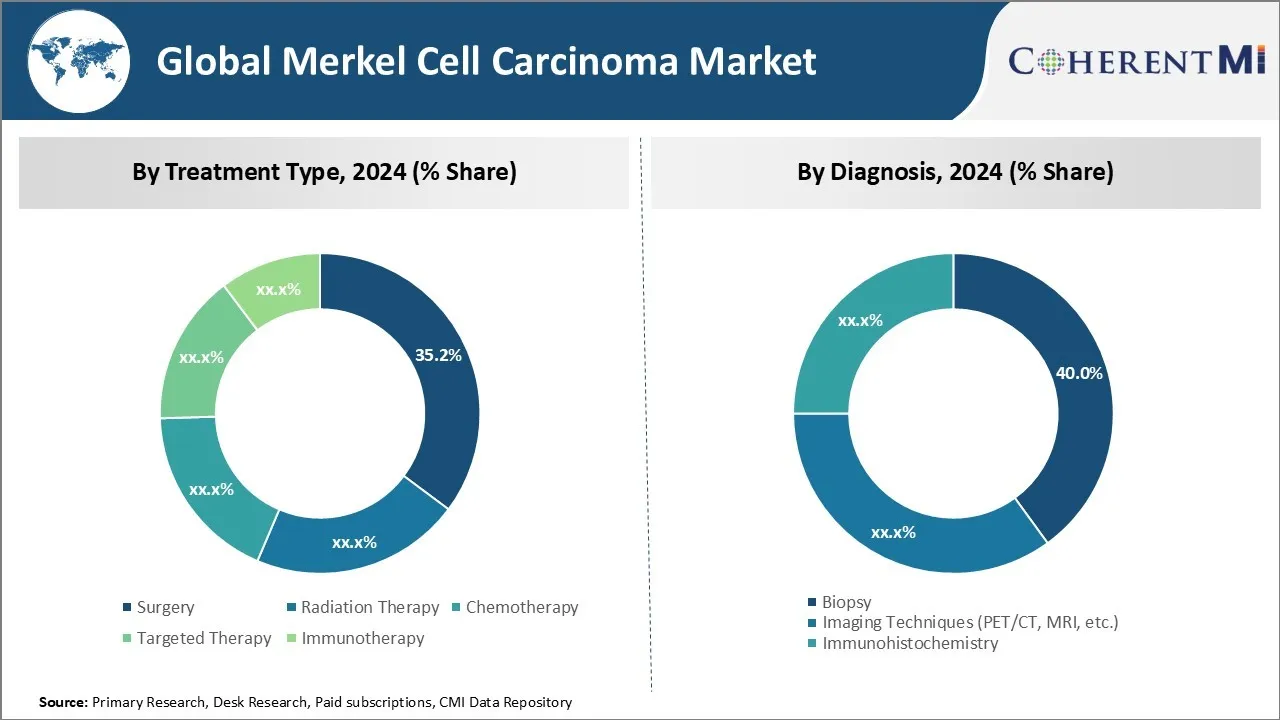
Insights, By Diagnosis - Biopsy Generates Highest Diagnostic Share Due to Accuracy
In terms of By Diagnosis, Biopsy contributes the highest share of the global Merkel cell carcinoma market with 40% in 2024 Biopsy is a diagnostic procedure that involves surgically removing tissue samples from suspicious lesions for microscopic examination. It plays a crucial role in definitively diagnosing Merkel cell carcinoma and distinguishing it from other skin malignancies. Unlike imaging techniques that only provide morphological data, biopsy allows for histopathological analysis of resected tissue to look for specific cellular markers that are characteristic of Merkel cell carcinoma. This includes examining the cells under a microscope for morphological patterns as well as conducting immunohistochemistry assays to detect Merkel cell polyomavirus antigens that are highly specific to this cancer. The high accuracy of biopsy in confirming Merkel cell carcinoma diagnoses has made it the gold standard approach worldwide. Its minimally invasive nature also enables biopsies to be performed quickly on easily accessible skin lesions. These advantages over competing diagnostic modalities contribute significantly to biopsy having the largest share in the global Merkel cell carcinoma diagnostics segment.
Insights, By Stage- Stage I Holds Dominant Share Due to Early Detection Focus
In terms of By Stage, Stage I contributes the highest share of the global Merkel cell carcinoma market with 42.3% in 2024. This can be attributed to growing efforts to detect the disease at its earliest stages through increased skin cancer screenings. Stage I Merkel cell carcinoma involves localized tumors that are generally 2cm or smaller in size and restricted to the epidermis or upper dermis layers of the skin. Early stage disease has a far superior 5-year survival rate of over 90% compared to advanced stages when the cancer has spread to local lymph nodes or distant organs. Therefore, recent years have witnessed heightened efforts by dermatologists and healthcare organizations to educate the public on self-skin examinations and increase participation in total body skin cancer screenings. This is raising awareness to detect abnormal or changing mole lesions at an early stage when surgical removal can easily cure the disease. Widespread screening initiatives have also led to incidental discovery of small stage I tumors during examinations for unrelated skin issues. Combined with evolving diagnostic techniques, these factors are contributing to more Merkel cell carcinomas now being diagnosed at the early stage I level versus later stages. This dominant share makes stage I an important segment of focus for the global market.
Additional Insights of Merkel Cell Carcinoma Market
Merkel Cell Carcinoma (MCC) is a rare but highly aggressive skin cancer with neuroendocrine features. It is primarily associated with Merkel cell polyomavirus (MCPyV) or chronic ultraviolet (UV) exposure. MCC presents as a rapidly growing cutaneous or subcutaneous tumor in sun-exposed areas, particularly the head and neck. The disease's aggressiveness is reflected in its staging, with clinical stages ranging from in situ (stage 0) to metastatic disease (stage IV). Treatment options include radiotherapy, systemic therapy, and immunotherapy, with radiotherapy being notably effective in controlling the disease in a significant percentage of cases. Immunotherapy has emerged as a superior option for advanced-stage disease, although not all patients respond to treatment. The market for MCC is expected to grow due to advancements in therapy, increasing awareness, and the introduction of new drugs, although challenges such as high treatment costs and limited specialist availability remain.
Competitive overview of Merkel Cell Carcinoma Market
The major players operating in the Global Merkel Cell Carcinoma Market include Merck, Bristol-Myers Squibb, Incyte Corporation, Kartos Therapeutics, Inc., Amgen Inc., Pfizer Inc., BeiGene, OncoSec Medical Incorporated, Immune Design and Immunomic Therapeutics, Inc.
Merkel Cell Carcinoma Market Leaders
- Merck
- Incyte Corporation
- Kartos Therapeutics, Inc.
- Bristol-Myers Squibb
- Amgen Inc.
Merkel Cell Carcinoma Market - Competitive Rivalry, 2024

Merkel Cell Carcinoma Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Merkel Cell Carcinoma Market
- In April 2023 Merck’s KEYTRUDA received accelerated approval from the FDA for treating locally advanced or metastatic Merkel Cell Carcinoma in pediatric patients. This approval underscores the drug’s growing application and potential to improve patient outcomes in younger populations.
- On January 2024, Incyte Corporation announced positive results from a Phase III trial of Retifanlimab for Merkel Cell Carcinoma, showing significant improvements in progression-free survival. This development could lead to an expanded use of Retifanlimab in clinical practice
Merkel Cell Carcinoma Market Segmentation
- By Treatment Type
- Surgery
- Radiation Therapy
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- By Diagnosis
- Biopsy
- Imaging Techniques (PET/CT, MRI, etc.)
- Immunohistochemistry
- By Stage
- Stage I
- Stage II
- Stage III
- Stage IV
- By End User
- Hospitals
- Oncology Centers
- Specialty Clinics
- Academic and Research Institutes

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
What are the key factors hampering the growth of the Global Merkel Cell Carcinoma Market?
The high cost of treatment, limiting accessibility for some patients. and limited awareness and diagnosis rates, particularly in early stages are the major factor hampering the growth of the Global Merkel Cell Carcinoma Market.
What are the major factors driving the Global Merkel Cell Carcinoma Market growth?
The increasing incidence of merkel cell carcinoma due to aging population and uv exposure. and advancements in immunotherapy treatments improving patient outcomes. are the major factor driving the Global Merkel Cell Carcinoma Market.
Which is the leading Treatment Type in the Global Merkel Cell Carcinoma Market?
The leading Treatment Type segment is Surgery.
Which are the major players operating in the Global Merkel Cell Carcinoma Market?
Merck, Bristol-Myers Squibb, Incyte Corporation, Kartos Therapeutics, Inc., Amgen Inc., Pfizer Inc., BeiGene, OncoSec Medical Incorporated, Immune Design, Immunomic Therapeutics, Inc. are the major players.
What will be the CAGR of the Global Merkel Cell Carcinoma Market?
The CAGR of the Global Merkel Cell Carcinoma Market is projected to be 4.1% from 2024-2031.