Prosthetic Joint Infection Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Prosthetic Joint Infection Market is segmented By Product (Antibiotics-Loaded Cement Spacers, Oral Antibiotics, Intravenous Antibiotics), By Infection....
Prosthetic Joint Infection Market Size
Market Size in USD Mn
CAGR5.2%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 5.2% |
| Market Concentration | Medium |
| Major Players | Zimmer Biomet, Smith & Nephew, DePuy Synthes (Johnson & Johnson), Stryker, 3M Healthcare and Among Others. |
please let us know !
Prosthetic Joint Infection Market Analysis
The prosthetic joint infection market is estimated to be valued at USD 64.2 Mn in 2024 and is expected to reach USD 91.6 Mn by 2031, growing at a compound annual growth rate (CAGR) of 5.2% from 2024 to 2031. Increasing prevalence of hospital acquired infections is one of the factors driving the prosthetic joint infection market during the forecast period.
Prosthetic Joint Infection Market Trends
Market Driver - Rise in the Aging Population Leading to More Joint Replacement Surgeries
For many aging individuals, joint disorders have progressed to an advanced stage where conservative treatments such as medication and physical therapy do not provide adequate relief anymore. This makes surgery the most viable option to get back the flexibility and improve quality of life, making it a growth prospect of growth for prosthetic joint infection market.
While joint replacement procedures have a high success rate of alleviating symptoms, the surgical site is always at risk of potential infections in the post-operative period. Elderly patients are additionally vulnerable as age-related factors can decrease the body's ability to fight infections. Even minor surgical site contaminations may progress into full-blown joint infections in these high-risk cases.
Therefore, as the percentage of senior citizens undergoing knee and hip replacements rises, it will continue to drive growth of the prosthetic joint infection market.
Market Driver - Advancements in Prosthetic Materials and Infection Prevention Techniques
Over the last decade, major technological advancements have been made in the field of orthopedic joint replacement implants as well as infection control practices adopted in operating rooms. Newly developed biomaterials used for producing prosthetic components offer better biocompatibility and resistance to bacterial biofilm formation compared to earlier generation implants.
On the other hand, strict adherence to evidence-based infection prophylaxis protocols during surgery has significantly lowered the rates of surgical site contamination. Application of antibiotic cement spacers also helps eliminate infection in already infected joints prior to revision procedures.
However, as some of these new interventions are still being validated through long-term data, prosthetic joint infections will remain an ongoing concern. Overall, innovations in biomaterials and safety protocols have opened up new possibilities in the prosthetic joint infection market for improved patient outcomes but also contribute to the persistent need for effective anti-infective therapies.
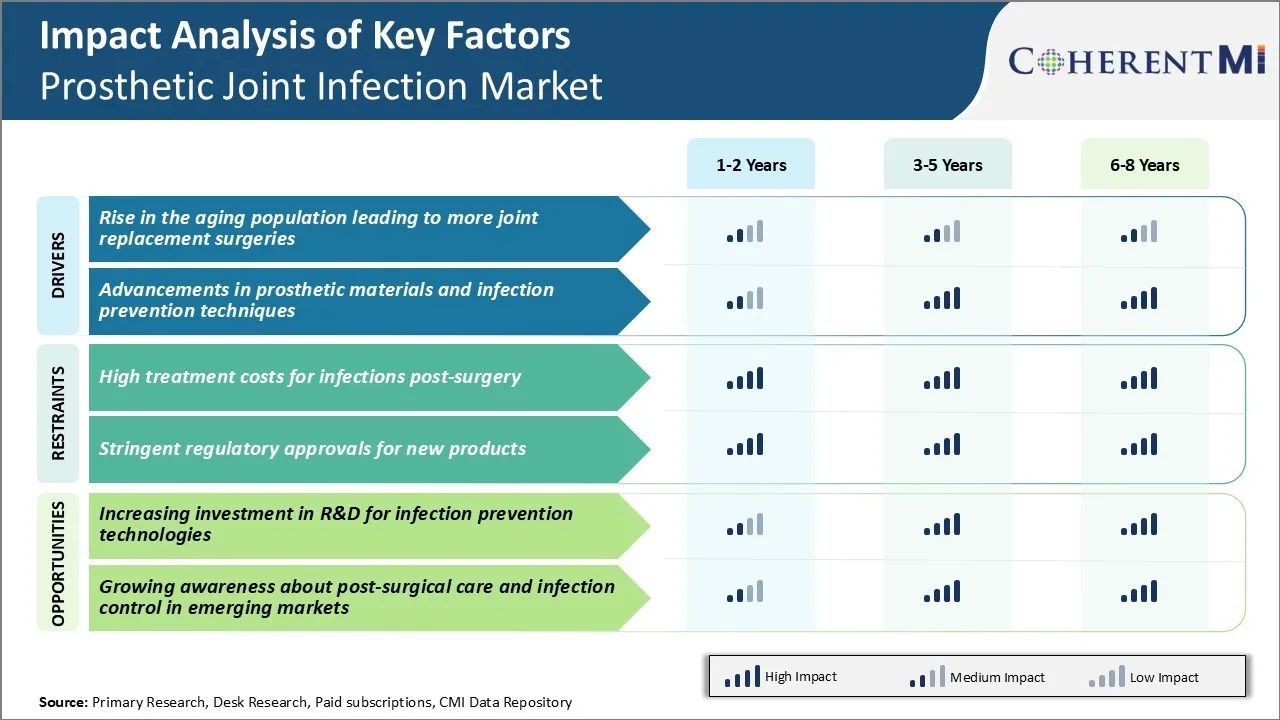
Market Challenge - High Treatment Costs for Infections Post-surgery
One of the key challenges faced by the prosthetic joint infection market is the high costs associated with treating infections that occur post-surgery. Prosthetic joint infections, such as those in hips and knees, can develop months or even years after the initial implantation procedure.
The average cost of treating a single case of prosthetic joint infection in the US is estimated to be over $50,000. This does not even account for lost work and wage costs for the patient. The financial burden is even higher for infections that are difficult to eradicate and require multiple surgical revisions and long antibiotic therapy.
As the number of joint replacement surgeries rise, especially among younger and more active individuals, the risk and incidence of infections is also increasing. This will significantly drive up the overall healthcare costs related to prosthetic joint infections. Unless new preventive and therapeutic solutions are discovered, treatment expenditures are projected to grow rapidly.
Market Opportunity - Increasing Investment in R&D for Infection Prevention Technologies
One promising opportunity for the prosthetic joint infection market lies in the rising investments and R&D activities focused on developing advanced infection prevention technologies. Companies and research institutes are investing more resources in areas such as antibiotic-eluting implants and biocompatible coatings that reduce bacterial adhesion.
For instance, there is ongoing research on coating joint implants with silver, antibiotics or host-defense peptides to prevent bacteria from latching on to the implant surface. Development of novel implant surfaces that stimulate soft tissue integration and wound healing can also aid infection prevention post-surgery.
With the projection of increasing rates of joint replacement procedures globally, vendors stand to gain significantly by introducing novel solutions that help lower infection risks and drive better patient outcomes. This will not only improve quality of life for patients but also help control escalating annual infection treatment costs.
Prescribers preferences of Prosthetic Joint Infection Market
For acute postoperative infections (PJI) presenting within 3 months of the original joint replacement surgery, prescribers commonly start with intravenous antibiotics like cefazolin (Ancef) administered in the hospital setting.
If the infection does not improve after 2-3 weeks, revisions surgery to remove the prosthesis and implant spacers is often considered. For chronic infections presenting more than 3 months post-surgery, prescribers may opt for prolonged suppressive oral antibiotic therapy using medications like rifampin (Rifadin) combined with a fluoroquinolone like levofloxacin (Levaquin).
In cases where the prosthesis needs to be removed, prescribers scrutinize the patient's risk factors like diabetes or previous infections to determine the optimal timing for re-implantation. This usually involves 4-6 weeks of intravenous antibiotics before a second revision surgery is performed to insert a new prosthesis.
Between surgeries, refillable antibiotic cement spacers may be utilized on an outpatient basis. For resistant infections, prescribers also consider specialized intravenous antibiotics like daptomycin (Cubicin) or ceftaroline (Teflaro) in combination therapy regimens.
Treatment Option Analysis of Prosthetic Joint Infection Market
For early PJI occurring within 3 months of joint replacement, debridement with implant retention and a long course of intravenous antibiotics is often recommended. Commonly used IV antibiotics include nafcillin/cefazolin for methicillin-sensitive infections and vancomycin for methicillin-resistant cases. This approach aims to save the prosthesis without complete removal.
Delayed PJI occurring 3-24 months post-surgery usually requires a two-stage revision, where the prosthesis is removed in the first stage along with debridement and placement of an antibiotic-loaded cement spacer. IV antibiotics are administered for 4-6 weeks. In the second stage, the spacer is removed and a new prosthesis implanted once the infection clears.
Late PJI beyond 24 months typically necessitates a one-stage or two-stage revision based on patient and infection factors. For polymicrobial or resistant infections, a two-stage treatment with prolonged antibiotics is preferred to achieve eradication. Antibiotic combinations involving rifampin, daptomycin or ceftaroline may be used along with standard antibiotics.
Key winning strategies adopted by key players of Prosthetic Joint Infection Market
Product innovation and expansion: One of the most common and important strategies adopted by key players has been continuous innovation and expansion of their product portfolio. For example, Zimmer Biomet launched the ROSA Knee System in 2018 which uses robotic-arm assisted surgery to implant prosthetic knees.
Strategic acquisitions: Companies have strengthened their position in prosthetic joint infection market and offerings by acquiring other established players. For example, in 2018, Stryker acquired K2M Group Holdings to expand its spine and extremities product portfolio.
Focus on infections solutions: With prosthetic joint infections posing a major challenge, providers have emphasized solutions to prevent and treat such complications. For example, Zimmer Biomet launched the INFINITY hip and kneecups with antimicrobial protection in 2015.
Partnerships & collaborations: Leveraging the expertise of other players, companies have entered partnerships for areas like research, manufacturing and geographical expansion. For example, in 2014, Wright Medical Group partnered with Anthropic to incorporate advanced AI capabilities into its total ankle replacement implants.
Segmental Analysis of Prosthetic Joint Infection Market
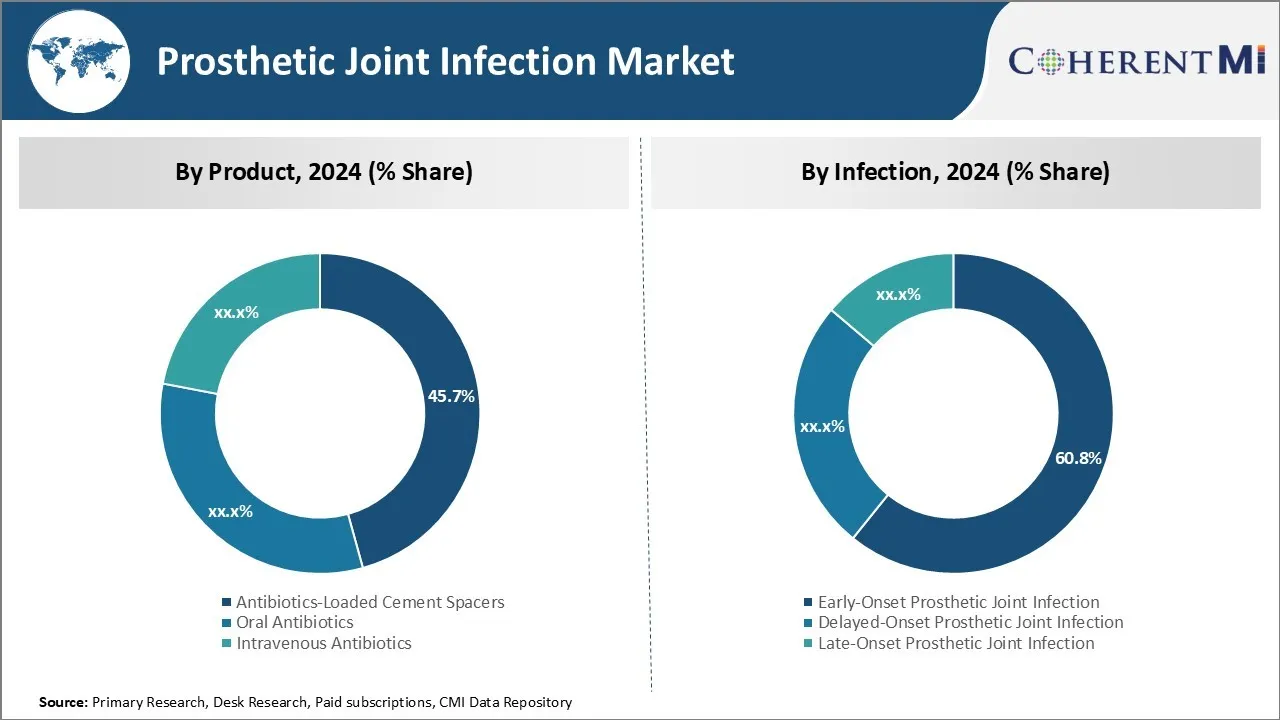
Insights, By Product: Effective Prevention and Treatment Drives Demand for Antibiotics-loaded Cement Spacers
In terms of product, antibiotics-loaded cement spacers are projected to account for 45.7% share of the prosthetic joint infection market in 2024. This is due to its effectiveness in preventing and treating prosthetic joint infections. These spacers are utilized during revision surgery after implanting a new joint implant. The antibiotics work to eliminate any residual bacteria and prevent recurrence of the infection.
Antibiotics-loaded cement spacers offer several advantages over intravenous or oral antibiotics alone. They allow for high concentrations of antibiotics to be directly delivered to the infected site rather than relying on systemic distribution. This targeted treatment enhances antibiotic efficacy and reduces the risk of re-infection or treatment failure.
The rise in joint replacement surgeries along with the inherent risk of post-operative infections has increased demand for effective infection management solutions. This is driving growth of the prosthetic joint infection market.
Insights, By Infection: High Risk of Early Infection Onset Drives Treatment of Early-Onset Prosthetic Joint Infection
In terms of infection, early-onset prosthetic joint infection is expected to account for 60.8% share of the prosthetic joint infection market in 2024. This is due to the high-risk factors associated with infection occurring within 3 months of initial implantation.
Treating early-onset infection promptly is therefore crucial to prevent irreversible joint destruction and implant failure. It requires lengthy use of high dose intravenous antibiotics along with irrigation and debridement surgeries to thoroughly wash out the infected site. Antibiotics-Loaded Cement Spacers are often implanted during revision to deliver targeted antibiotic therapy directly to the joint. Single or multiple-stage reimplantation strategies are then decided based on infection clearance signs.
However, the surgery and hospital environment pose an intrinsic danger of early bacterial contamination. Advances in preventive measures and new diagnostic tools that allow for faster pathogen identification are contributing to higher early-onset prosthetic joint infection segment growth. Better infection control protocols and antibiotic prophylaxis are also helping lower the risks of early infection onset.
Insights, By End User: Infrastructure and Resources Drive Hospital Preference for Treatment
In terms of end user, hospitals contribute the highest share of the prosthetic joint infection market owning to their extensive infrastructure and resources well-equipped to handle complex prosthetic joint infections. Treating established joint infections requires diligent long-term antibiotic management along with possible revision surgeries for implant removal and replacement. This sophisticated treatment protocol is best supported in a hospital setting.
Hospitals have dedicated orthopedic wings and operating rooms with specialized joint replacement surgeons. They offer 24/7 emergency and critical care necessary during infection flare-ups or post-surgical complications. Large hospitals also have robust microbiology departments that allow for timely pathogen identification and antibiotic sensitivity testing.
As joint infections become more resistant to treatment, the hospital environment will remain crucial in the prosthetic joint infection market. However, complementary roles for ambulatory centers in providing certain outpatient antibiotic therapies or minor debridement could help reduce hospital stays in the future.
Additional Insights of Prosthetic Joint Infection Market
- In 2023, there were approximately 89,176 total cases of prosthetic joint infection across the 7MM, with the US accounting for the highest proportion at 63%.
- Gender-specific cases in Japan showed 1,491 cases in males and 1,250 in females.
- Prosthetic joint infections caused by a variety of pathogens, including gram-positive and gram-negative bacteria, require a multi-faceted treatment approach due to complexities in biofilm formation and antibiotic resistance.
- Early and accurate diagnosis via advanced techniques is crucial for improving treatment outcomes.
- Prosthetic joint infection cases are expected to increase by 25% over the next decade. Additionally, the introduction of biofilm-targeting antibiotics is projected to reduce infection rates by 15%.
Competitive overview of Prosthetic Joint Infection Market
The major players operating in the prosthetic joint infection market include Zimmer Biomet, Smith & Nephew, DePuy Synthes (Johnson & Johnson), Stryker, 3M Healthcare, Osteal Therapeutics, Peptilogics, TenNor Therapeutics, and Arrevus.
Prosthetic Joint Infection Market Leaders
- Zimmer Biomet
- Smith & Nephew
- DePuy Synthes (Johnson & Johnson)
- Stryker
- 3M Healthcare
Prosthetic Joint Infection Market - Competitive Rivalry, 2024

Prosthetic Joint Infection Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Prosthetic Joint Infection Market
- In August 2024, Osteal Therapeutics announced promising results from two APEX trials for the VT-X7 KIT, showcasing potential to revolutionize prosthetic joint infection market with enhanced recovery rates. The VT-X7 KIT, designed for treating periprosthetic joint infections, demonstrated a significant improvement in recovery rates compared to the standard two-stage exchange arthroplasty. The results showed that patients treated with VT-X7 experienced a net treatment effect of 41%, with faster recovery times—achieving the second stage of surgery in just seven days compared to 102 days for the control group.
- In July 2024, Zimmer Biomet launched a new generation of antibiotics-loaded cement spacers, offering better infection control and easier integration with existing prosthetics. These spacers are designed to enhance infection control during two-stage revision surgeries, particularly for treating periprosthetic joint infections (PJIs). The new generation spacers offer improved integration with existing prosthetic systems, allowing for easier placement and removal, while delivering localized antibiotic therapy to reduce infection risks. This launch reflects Zimmer Biomet's ongoing efforts to innovate in the prosthetic joint infection market.
- In May 2023, Stryker collaborated with leading orthopedic surgeons to develop a new line of modular prosthetics aimed at reducing infection rates during and post-surgery.
Prosthetic Joint Infection Market Segmentation
- By Product
- Antibiotics-Loaded Cement Spacers
- Oral Antibiotics
- Intravenous Antibiotics
- By Infection
- Early-Onset Prosthetic Joint Infection
- Delayed-Onset Prosthetic Joint Infection
- Late-Onset Prosthetic Joint Infection
- By End User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
How big is prosthetic joint infection market?
The prosthetic joint infection market is estimated to be valued at USD 64.2 Mn in 2024 and is expected to reach USD 91.6 Mn by 2031.
What are the key factors hampering the growth of the prosthetic joint infection market?
High treatment costs for infections post-surgery and stringent regulatory approvals for new products are the major factors hampering the growth of the prosthetic joint infection market.
What are the major factors driving the prosthetic joint infection market growth?
Rise in the aging population leading to more joint replacement surgeries and advancements in prosthetic materials and infection prevention techniques are the major factors driving the prosthetic joint infection market.
Which is the leading product in the prosthetic joint infection market?
The leading product segment is antibiotics-loaded cement spacers.
Which are the major players operating in the prosthetic joint infection market?
Zimmer Biomet, Smith & Nephew, DePuy Synthes (Johnson & Johnson), Stryker, 3M Healthcare, Osteal Therapeutics, Peptilogics, TenNor Therapeutics, and Arrevus are the major players.
What will be the CAGR of the prosthetic joint infection market?
The CAGR of the prosthetic joint infection market is projected to be 5.2% from 2024-2031.