Severe Asthma Drugs Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Severe Asthma Drugs Market is segmented By Current Therapies (Inhaled Corticosteroids, Biologic Therapies, Bronchodilators), By Biologic Therapies (IL....
Severe Asthma Drugs Market Size
Market Size in USD Bn
CAGR6.1%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 6.1% |
| Market Concentration | High |
| Major Players | GlaxoSmithKline (GSK), Biosion, Bio-Thera Solutions, Sanofi, AstraZeneca and Among Others. |
please let us know !
Severe Asthma Drugs Market Analysis
The Global Severe Asthma Drugs Market is estimated to be valued at USD 24.51 Bn in 2024 and is expected to reach USD 40.66 Bn by 2031, growing at a compound annual growth rate (CAGR) of 6.1% from 2024 to 2031. Several key factors such as the growing prevalence of asthma worldwide and rising pollution levels are expected to drive the demand for severe asthma drugs during the forecast period.
The Severe Asthma Drugs Market is expected to witness significant growth over the next few years. The market is currently dominated by biologics and there is high demand for novel drug molecules that are more effective and have fewer side effects. Many pharmaceutical companies are actively involved in research & development to develop advanced therapeutics, which is likely to intensify competition in the market.
Severe Asthma Drugs Market Trends
Market Driver - Rising Prevalence of Severe Asthma Due to Increasing Environmental Pollutants.
With rapid industrialization and urbanization, environmental pollution has become a major issue impacting public health. While outdoor air pollution stems from vehicular emissions, construction activities, power plants indoor air quality is also compromised due to rising use of chemicals, furniture, construction materials and household products that emit toxic fumes. Severe asthmatics are especially vulnerable to effects of pollutants as their lung function is already compromised.
Studies show a direct correlation between rising levels of particulate matter (PM 2.5 and PM 10), ozone, nitrogen dioxide, sulfur dioxide and prevalence of asthma cases especially among children and elderly. These pollutants have the ability the penetrate deeper into the lungs and even bloodstream, causing inflammatory responses. Repeated exposure over time damages the airways and lungs, deteriorating lung function in asthmatics. Allergens and irritants from polluted air also act as triggers, aggravating asthma symptoms. Incidents of acute exacerbations leading to emergency room visits and hospitalizations have seen a conspicuous rise parallel to worsening air quality.
Market Driver - Growing Advancements in Biologics and Monoclonal Antibodies for Personalized Treatments.
The development of targeted biological drug therapies heralds a new era for specialized treatment of severe refractory asthma. Conventional asthma controllers are often inadequate to rein in uncontrolled symptoms among severe asthmatics, calling for novel pharmacological approaches. Monoclonal antibodies that block specific inflammatory pathways implicated in asthma pathology have shown great promise.
Drugmakers are continuously advancing science to develop highly selective biologics attacking molecular triggers of asthma. Compared to traditional 'one size fits all' therapies, these biologics enable personalized care by precisely interfering with Asthma's specific pathological processes in each patient. Drugs inhibiting mediators like IgE, IL-5, IL-4Rα have proved dramatically effective in reducing exacerbations for certain asthma phenotypes. Currently approved mAbs like omalizumab, mepolizumab, reslizumab, benralizumab continue to transform management of severe eosinophilic and allergic asthma.
The booming pipeline includes several new paradigms for targeting emerging inflammatory pathways and phenotypes. These encompass antibodies against IL-33, IL-13, TSLP among others. Combined therapies are also envisioned to offer synergistic Clinical benefits. A major fillip is that biologics are well-tolerated and demonstrate long-term safety. Novel delivery routes now facilitate self-administration outside healthcare settings. These advantages will help escalate the uptake of biologics for asthma care, driving the accompanying market.
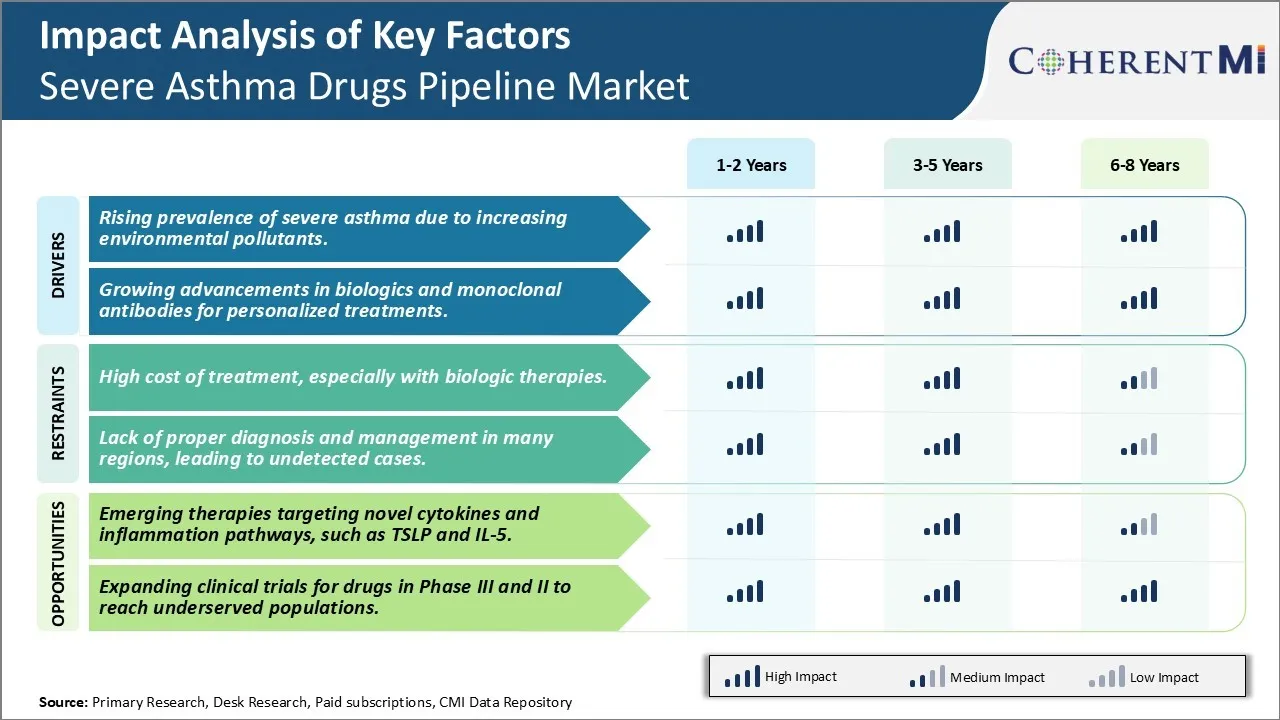
Market Challenge - High Cost of Treatment, Especially with Biologic Therapies.
One of the major challenges currently facing the Severe Asthma Drugs Market is the high cost of treatment associated with existing and emerging biologic therapies. Biologics such as monoclonal antibodies that target specific cytokines and inflammatory pathways have revolutionized the treatment of severe refractory asthma over the past decade. However, these biologic drugs often have list prices in the range of USD 20,000 to USD 50,000 per patient annually. The high cost of biologics has imposed significant economic burden on patients as well as healthcare systems globally. Out-of-pocket costs after insurance can still be prohibitive for many patients. Moreover, the high resource requirements to administer biologic injections also increases treatment costs. The sustainability of the severe asthma market is threatened by the increasing pharmaceutical expenditures on high-priced treatments. Drug developers will need to explore strategies such as outcome-based pricing models and expanded patient access programs to make novel therapies more affordable and increase their uptake. However, balancing treatment innovation with cost containment remains a major challenge for the future growth of this market segment.
Market Opportunity - Emerging Therapies Targeting Novel Cytokines And Inflammation Pathways, Such as TSLP and IL-5.
One of the key opportunities for the severe asthma drugs market lies in emerging therapeutics that target novel cytokines and inflammatory pathways implicated in disease pathogenesis. There is ongoing research focused on developing drugs against newer targets such as thymic stromal lymphopoietin (TSLP) and interleukin-5 (IL-5). TSLP has emerged as an important epithelial cytokine that promotes type 2 inflammation. Drugs blocking TSLP signaling could provide an effective treatment option for a significant subset of severe asthma patients. Similarly, targeting the cytokine IL-5, which is a key driver of eosinophilic inflammation, with monoclonal antibodies is a promising novel therapeutic approach. Drugs targeting these and other emerging inflammatory pathways have the potential to significantly enhance therapeutic efficacy for severe asthma compared to existing standard-of-care options. Their selective mechanisms of action may also result in improved safety profiles. This could translate to better patient outcomes and higher treatment compliance. The introduction of effective therapies against novel drug targets is expected to fuel significant growth in the severe asthma drugs market over the next decade.
Prescribers preferences of Severe Asthma Drugs Market
Asthma is generally treated through a step-wise approach depending on the severity and frequency of symptoms. For mild intermittent asthma with symptoms less than twice a week, short-acting beta-agonists (SABA) like Salbutamol (Ventolin) are prescribed on an as-needed basis.
If symptoms occur more than twice a week, low-dose inhaled corticosteroids (ICS) are added for persistent asthma. Common ICS drugs include Beclometasone (Qvar), Budesonide (Pulmicort) and Fluticasone propionate (Flixotide). These are generally combined with long-acting beta-agonists (LABA) like Salmeterol (Serevent) or Formoterol (Oxeze) for better asthma control.
For moderate persistent asthma with daily symptoms, medium-dose ICS-LABA combinations are prescribed. Popular choices are Seretide/Advair which combine Salmeterol and Fluticasone, and Foster which combines Formoterol and Budesonide. Oral corticosteroids like Prednisolone may be added for short term relief during exacerbations.
In severe persistent cases, high-dose ICS-LABA or leukotriene receptor antagonists like Montelukast (Singulair) along with oral corticosteroids are commonly used. Maximizing inhaler adherence through education is also important. Key factors influencing prescribers include clinical guidelines, cost, insurance coverage, safety profile, incidence of side effects and patient's preference based on inhaler device familiarity.
Treatment Option Analysis of Severe Asthma Drugs Market
Asthma is typically classified into 4 stages - mild intermittent, mild persistent, moderate persistent, and severe persistent - based on symptoms and lung function tests. For mild intermittent asthma, short-acting beta2 agonists (SABA) like albuterol are used as needed for symptom relief. If symptoms occur more than 2 days a week, inhaled corticosteroids (ICS) like fluticasone become the standard first-line therapy. For mild persistent asthma, low-dose ICS are recommended either as monotherapy or along with leukotriene receptor antagonists (LTRA) like montelukast. Combining low-dose ICS with LABA like salmeterol is more effective than increasing the ICS dose alone for better symptom control and fewer exacerbations.
Moderate persistent asthma requires low-to-medium dose ICS-LABA combinations like fluticasone-salmeterol. Medium-dose ICS-LABA combinations such as budesonide-formoterol are also used, providing a fast onset of action within 10-15 minutes.
Finally, for severe persistent asthma, high-dose ICS-LABA combinations like beclomethasone-formoterol are recommended. Oral corticosteroids may also be added to this regimen to gain control during exacerbations. Biologic antibodies targeting cytokines like omalizumab are preferred for those with allergies.
The treatment choice corresponds to the disease severity and aims to provide the maximum benefit with minimal side effects through step-wise escalation of therapy when control deteriorates. The branded combination inhalers offer convenience of therapy and better adherence.
Key winning strategies adopted by key players of Severe Asthma Drugs Market
Focus on Developing Biologics and Novel Mechanism Drugs: Many large pharmaceutical companies such as GSK, AstraZeneca, Teva etc. are focusing their R&D efforts on developing biologics such as monoclonal antibodies (mAbs) and other novel mechanism drugs to target specific pathways involved in severe asthma. For example, AstraZeneca is developing a novel drug targeting the IL-33 pathway which is in late-stage trials. Targeting novel pathways instead of existing mechanism classes helps companies gain regulatory exclusivity and block competition.
Partnering/Licensing Deals to Access New Candidates: Companies often lack internal pipeline depth and look to partnering/licensing deals to gain access to new clinical/pre-clinical stage candidates from smaller biotechs. For example, in 2019, AstraZeneca partnered with Hookipa Pharma to develop viral-vector based immunotherapies for asthma. Similarly, Teva partnered with Theravance to develop an oral mAb for severe asthma. Such deals reduce development risks and help launch products faster.
Focus on Patient Segmentation Studies: Companies are increasingly segmenting severe asthma patients into different endotypes/phenotypes based on triggers, and biomarkers to identify specific sub-groups most likely to respond to certain drugs. GSK's 2019 acquisition of scientists from Grail is aimed at using multiomics to better segment patients. Such precision medicine approaches will help target the right drugs to the right patients and maximize commercial success.
Expanding into new Geographies: While developed markets are saturated, companies are now expanding into growth markets like China, Brazil, India by partnering with local players who handle regulatory/commercial aspects.
Segmental Analysis of Severe Asthma Drugs Market
Insights, By Current Therapies, Rising Prevalence of Asthma Symptoms Drives Demand for Inhaled Corticosteroids.
By Current Therapies, Inhaled Corticosteroids is expected to contribute the highest share 55.2% in 2024 owing to their effectiveness in controlling asthma symptoms. Asthma is a chronic inflammatory disease of the airways characterized by symptoms like wheezing, coughing, chest tightness and shortness of breath. The prevalence of asthma has been steadily increasing over the past few decades, primarily due to environmental pollution, sedentary lifestyles and poor dietary habits. According to research, over 300 million people worldwide suffer from asthma today, with nearly 25 million cases reported in the United States alone.
Inhaled corticosteroids are considered the most effective long-term treatment option for persistent asthma as they work by reducing inflammation in the airways. These drugs when taken regularly as preventive therapy help gain good control over asthma symptoms and reduce the risk of attacks and emergencies. The growing patient pool of both adults and children experiencing frequent asthma attacks and symptoms on a long-term basis has majorly contributed to the higher demand and uptake of inhaled corticosteroids. Their easy and convenient mode of administration through inhalers also makes these drugs the preferred first line therapy for asthma patients over other traditional options.
Manufacturers are also focusing on developing more user-friendly inhaler devices and formulations of existing inhaled corticosteroids to improve medication adherence. The approval and launch of combo inhalers containing both corticosteroids and long-acting bronchodilators have further improved treatment outcomes. Supported by strong clinical evidence of efficacy and safety, inhaled corticosteroids will continue dominating the asthma drugs market over the forecast period.

Insights, By Biologic Therapies, High Disease Burden of Eosinophilic Asthma Fuels Growth of IL-5 Inhibitors.
By Biologic Therapies, IL-5 Inhibitors is expected to contribute the highest share 48.7% in 2024 owing to their superior efficacy in managing eosinophilic asthma. Eosinophilic asthma is a severe type characterized by elevated levels of eosinophils, a type of white blood cell, in the airways, sputum or blood of patients. These cells play a central role in triggering inflammation and symptoms in around 40-60% of adult asthma patients. Existing asthma therapies often fail to gain complete control over symptoms in such refractory cases with significant eosinophilic inflammation.
The approval of the first IL-5 targeting monoclonal antibodies like Mepolizumab and Reslizumab proved highly beneficial for these difficult-to-treat patients. By inhibiting IL-5, a key driver of eosinophil development, maturation and activation, these biologics are able to deplete eosinophil counts significantly. This results in better asthma management for patients with frequent exacerbations despite treatment with high-dose inhaled or oral corticosteroids. IL-5 inhibitors also allow reduction in maintenance oral corticosteroid doses. With no well-established alternative treatment options, the demand for IL-5 targeting drugs is mounting given their ability to minimize asthma attacks and symptoms in cases unresponsive to conventional therapy.
Insights, By Mode of Administration, Tablets and Capsules Emerge as Most Convenient Mode of Administration
By Mode of Administration, tablets and capsules contributes the highest share of the market owing to patient preference for oral medications over other modes. While inhalers remain the first line of delivery for asthma drugs, many patients find it challenging to use pressurized metered-dose and dry powder inhaler devices correctly and regularly. This is a key cause behind poor symptom control and medication non-adherence seen frequently. However, with tablets and capsules, patients enjoy hassle-free oral dosing without having to coordinate inhalation or deal with device-related issues.
The dominance of tablets is expected to grow further with the approval of new once-daily oral drugs in the pipeline for asthma. These single-dose long-acting maintenance drugs promise around-the-clock symptom relief and may replace the need for multiple inhalers throughout the day. The growing elderly asthma population also prefers oral medications over complex inhaler regimens due to ease of use. Liquid medication alternatives have also gained ground to facilitate use in pediatric patients. Thus, the asthma drugs market is shifting towards more user-friendly oral options to boost treatment adherence and overcome challenges with inhaler technologies.
Additional Insights of Severe Asthma Drugs Market
The severe asthma drugs market is evolving with the development of targeted therapies that address the underlying inflammatory processes of the disease. Monoclonal antibodies like GSK’s GSK3511294 and Biosion’s BSI-045B are focusing on IL-5 and TSLP pathways, respectively, offering personalized treatment options for patients with difficult-to-treat asthma. The key challenge in managing severe asthma is controlling inflammation that is resistant to conventional treatments, which often leads to frequent exacerbations and hospitalizations. Biologic drugs, which modulate immune pathways, have shown promise in reducing symptoms and preventing exacerbations, but their high cost remains a barrier to widespread adoption. Clinical trials are expanding across the globe to test these drugs in diverse populations, and their success could lead to more affordable and accessible treatments, improving quality of life for severe asthma patients.
Competitive overview of Severe Asthma Drugs Market
The major players operating in the Severe Asthma Drugs Market include GlaxoSmithKline (GSK), Biosion, Bio-Thera Solutions, Sanofi, AstraZeneca, Merck & Co. Inc, Novartis, Amgen and Johnson & Johnson.
Severe Asthma Drugs Market Leaders
- GlaxoSmithKline (GSK)
- Biosion
- Bio-Thera Solutions
- Sanofi
- AstraZeneca
Severe Asthma Drugs Market - Competitive Rivalry, 2024

Severe Asthma Drugs Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Severe Asthma Drugs Market
- In August 2024, GlaxoSmithKline’s GSK3511294 (depemokimab) is progressing through Phase III trials. This monoclonal antibody targets IL-5 and is designed for long-term management of severe asthma with an eosinophilic phenotype. This development aims to offer long-acting treatment with less frequent dosing, improving patient compliance and outcomes.
- In June 2024, Biosion’s BSI-045B, a monoclonal antibody targeting thymic stromal lymphopoietin (TSLP), entered Phase II trials in China. This drug shows promise in reducing airway inflammation and improving lung function in patients with severe, uncontrolled asthma.
Severe Asthma Drugs Market Segmentation
- By Current Therapies
- Inhaled Corticosteroids
- Biologic Therapies
- Bronchodilators
- By Biologic Therapies
- IL-5 Inhibitors
- TSLP Inhibitors
- Cytokine Inhibitors
- By Mode of Administration
- Tablets and Capsules
- Liquid Inhalers
- Injections
- By Application
- Pediatric
- Adults

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
How big is the severe asthma drugs market?
The Global Severe Asthma Drugs Market is estimated to be valued at USD 24.51 Bn in 2024 and is expected to reach USD 40.66 Bn by 2031.
What will be the CAGR of the Severe Asthma Drugs Market?
The CAGR of the Severe Asthma Drugs Market is projected to be 6.1% from 2023 to 2031.
What are the major factors driving the Severe Asthma Drugs Market growth?
The rising prevalence of severe asthma due to increasing environmental pollutants and growing advancements in biologics and monoclonal antibodies for personalized treatments are the major factor driving the Severe Asthma Drugs Market.
What are the key factors hampering the growth of the Severe Asthma Drugs Market?
The high cost of treatment, especially with biologic therapies and lack of proper diagnosis and management in many regions, leading to undetected cases are the major factor hampering the growth of the Severe Asthma Drugs Market.
Which is the leading Current Therapies in the Severe Asthma Drugs Market?
Inhaled Corticosteroids is the leading Current Therapies.
Which are the major players operating in the Severe Asthma Drugs Market?
GlaxoSmithKline (GSK), Biosion, Bio-Thera Solutions, Sanofi, AstraZeneca, Merck & Co. Inc, Novartis, Amgen, Johnson & Johnson are the major players.