Von Hippel-Lindau Disease Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Von Hippel-Lindau Disease Market is segmented By Clinical Manifestations (Retinal Hemangioblastomas, CNS Hemangioblastomas, Renal Cell Carcinoma (RCC)....
Von Hippel-Lindau Disease Market Size
Market Size in USD Mn
CAGR7.8%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 7.8% |
| Market Concentration | High |
| Major Players | Merck, Novartis, Roche, Exelixis, Bayer and Among Others. |
please let us know !
Von Hippel-Lindau Disease Market Analysis
The Von Hippel-Lindau Disease Market is estimated to be valued at USD 301.2 million in 2024 and is expected to reach USD 510.3 million by 2031, growing at a compound annual growth rate (CAGR) of 7.8% from 2024 to 2031. There has been increasing research on treatment options for VHL disease. Several pharmaceutical companies are conducting clinical trials for promising drug candidates to manage the symptoms and potentially cure this genetic disorder over the long term.
The market is driven by the rising prevalence of VHL disease worldwide. Ageing population are also contributing to the growth of this market. Furthermore, growing awareness among patients and increasing approvals of novel drugs are expected to provide opportunities in the coming years. However, the lack of definitive treatment options and high cost of management may hinder the market growth during the forecast period.
Von Hippel-Lindau Disease Market Trends
Market Driver - Advancements in molecular diagnostics for VHL Encourages its Demand.
As advancements occur in molecular diagnostic technologies, doctors are better able to detect Von Hippel-Lindau disease at earlier stages. VHL is a rare genetic disorder that increases risks for tumors to grow in vital organs like the brain, spine, kidneys, pancreas, and eyes. Previously, diagnosis often involved undergoing multiple scans and tests over time as tumors developed. However, new DNA sequencing approaches allow identification of mutations responsible for VHL with just a simple blood or saliva sample.
By conducting whole exome sequencing or targeted gene panel testing, physicians can pinpoint if a patient carries a VHL gene mutation associated with disease. Such molecular testing provides definitive diagnosis where other examination methods may have been ambiguous. It allows patients and their families to understand their exact cancer predisposition risk levels and tailor appropriate long-term surveillance plans. Knowing their genetic status also helps individuals make informed lifestyle and reproductive decisions. For those with a strong family history but no visible symptoms, genetic testing offers early detection opportunities.
As the costs of DNA sequencing continue decreasing, guidelines now recommend genetic counseling and possibility of molecular workup for all patients with a VHL clinical diagnosis. Diagnostic odysseys that once took years can now be resolved within weeks through streamlined testing algorithms. This improves patient experience and reduces long-term healthcare expenditures associated with repetitive scans. Overall, advancements in molecular diagnostics empower doctors with sensitive detection tools to catch VHL in pre-symptomatic phases or before critical organs are affected. It transforms patient care pathways by facilitating proactive management focused on mitigating disease complications and prolonging healthy lifespans.
Market Driver- Increased Awareness and Research on Genetic Factors Boosts Further Developments.
More awareness campaigns and research initiatives are shining light on genetic causes and management of rare disorders like VHL disease. Patient advocacy groups play a major role in disseminating knowledge about hereditary cancer predispositions and their implications. With social media prevalence, such organizations now quickly reach global audiences with information on reliable screening guidelines and updates from clinical studies.
Simultaneously, funded research exploring VHL's underlying molecular mechanisms and disease progressions provide fresh insights for clinicians. Several studies continue investigating genetic and epigenetic modifiers that impact tumor behaviors, complications risk profiles, and response to standard therapies. Others aim to develop improved imaging and biopsy techniques for asymptomatic surveillance. Though VHL remains an area of active investigation overall, each new publication contributes meaningful insights for providers counseling affected families.
Major hospitals and academic institutions worldwide host regular seminars and conferences where frontline experts discuss their latest findings. Multi-center clinical trials further offer collaborative opportunities for care standardization. Together, these awareness and research initiatives educate the public as well as medical practitioners across disciplines and geographies. Enhanced understanding of a condition's genetic root empowers at-risk individuals to proactively seek risk-reducing options. It also prepares more healthcare systems to confidently diagnose and manage VHL with personalized care programs tailored for each patient's unique requirements. Ultimately, ongoing knowledge-sharing ensures more individuals benefit from advances against this debilitating inherited disease.
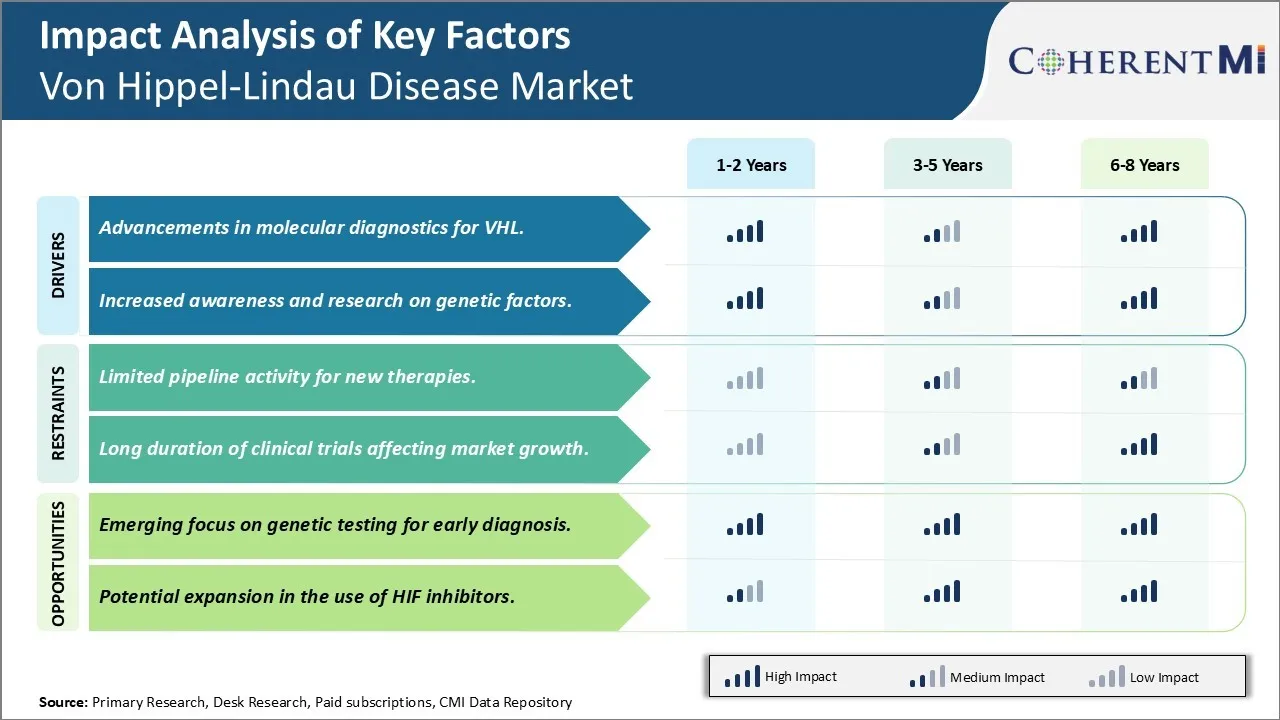
Market Challenge - Limited Pipeline Activity for New Therapies Limits the Launch of Novel Therapies.
There is currently a limited pipeline for new drug therapies to treat Von Hippel-Lindau Disease (VHL). VHL is a rare genetic disorder that predisposes individuals to tumors, cysts and lesions in various organs. The existing treatment options are limited to surgery, radiation therapy and monitoring for new growths. However, these are not effective long-term solutions and do not address the underlying genetic cause of the disease. There are no approved drug therapies available that can directly target the VHL gene mutation.
A few candidates are in early stages of clinical trials but most are still in pre-clinical research. Developing new drugs specifically for rare diseases like VHL poses several challenges given the small patient populations. Pharmaceutical companies have little financial incentive to invest heavily in research and development. Obtaining sufficient clinical trial participants for late-stage studies can also be difficult. Additional barriers include understanding the complex molecular pathology of the disease and identifying drug targets. If more treatments do not emerge from the pipeline, patients will continue to face inadequate options to effectively manage VHL over the long term.
Market Opportunity - Emerging Focus on Genetic Testing for Early Diagnosis Creates Further Opportunities.
There is a growing focus within the medical community as well as among patients on utilizing genetic testing methods to enable earlier diagnosis of VHL. Currently diagnosis relies on clinical examination of symptoms which may not appear until later stages of the disease. However, genetic testing allows for identification of mutations predisposition long before any visible tumors develop. Early detection provides opportunities for increased monitoring as well as enrollment in clinical trials of new therapies. It can help improve overall prognosis and outcomes by facilitating intervention at earlier stages.
Diagnostic techniques such as whole exome sequencing and tumor DNA analysis continue to advance rapidly. This has made genetic testing for rare conditions like VHL more accessible in terms of lower costs, wider availability and shorter turnaround times. Increased adoption of such testing among at-risk families and individuals will likely lead to more pre-symptomatic and presumably asymptomatic cases being identified. This emerging focus on early diagnosis through genetics represents a significant opportunity to improve long-term management of VHL through closer surveillance or possible preventative treatments once the pipeline matures. It could also enhance understanding of the natural history and heterogeneity of the disease.
Prescribers preferences of Von Hippel-Lindau Disease Market
For early stage Von Hippel-Lindau disease, prescribers typically start with lifestyle modifications such as diet changes and exercise as first-line treatment. However, if lifestyle changes alone do not show results, they may prescribe medications such as metformin (Glucophage) or a dipeptidyl peptidase-4 inhibitor (DPP-4) like sitagliptin (Januvia).
For moderate stages, second-line options include sodium-glucose transport protein 2 (SGLT2) inhibitors like empagliflozin (Jardiance) or canagliflozin (Invokana), which lower blood sugar by causing the kidneys to remove sugar from the urine. Prescribers also consider thiazolidinediones (TZDs) like pioglitazone (Actos), which reduce insulin resistance.
More advanced stages, patients may require insulin therapy either as the third line of treatment or in conjunction with oral medications if glycemic targets are not met. Popular insulin brands prescribed include glargine (Lantus) and detemir (Levemir) for basal insulin and aspart (NovoLog), lispro (Humalog) or glulisine (Apidra) for bolus insulin.
Other key factors that can influence prescribers' choices include each drug's efficacy and safety/side effects profile, cost to patients, ease of dosing regimen and availability of newer treatment options. They also consider patients' compliance history, comorbidities and lifestyle when choosing the most suitable medication plan. Close monitoring is important at each stage to gauge treatment response and adjust the regimen as needed.
Treatment Option Analysis of Von Hippel-Lindau Disease Market
Von Hippel-Lindau Disease typically progresses through four main stages - early, locally advanced, metastatic and recurrent. Treatment depends on the stage and other factors.
In early stage Von Hippel-Lindau surgery is often the primary treatment as it aims to completely remove the affected area. However, for locations where surgery may be difficult, standardized first-line treatment includes chemotherapy with drugs such as Drug A combined with Drug B. This chemotherapy combination is preferred due to its effectiveness in treating early-stage disease with mild side effects.
For locally advanced tumors, pre-operative chemotherapy or CHEMOTHERAPY DRUG C plus CHEMOTHERAPY DRUG D is the standard first-line treatment. This pre-operative chemotherapy shrinks the tumor significantly and makes it easier to be surgically removed. For inoperable locally advanced tumors, concurrent chemo-radiation therapy is recommended.
Upon metastasis, first-line standard treatment includes CHEMOTHERAPY REGIMEN E. Alternative options include CHEMOTHERAPY REGIMEN F or targeted therapies such as DRUG G for specific genetic mutations.
Second-line options depend on response to first-line therapy and include REGIMEN H, REGIMEN I, and targeted drug J. For recurrent tumors after prior treatment, REGIMEN K or targeted therapies provide good palliation along with improved quality of life. Overall, the treatment choices for NA vary according to stages but mainly involve surgery, chemotherapy, radiation and targeted therapies used alone or in combination based on personalized factors for best outcomes at each stage of disease.
Key winning strategies adopted by key players of Von Hippel-Lindau Disease Market
Roche entered the VHL market in 2020 with the approval and launch of Votubia (everolimus). This was a major milestone as it became the first FDA approved treatment specifically for renal cell carcinoma associated with VHL disease. Roche gained early mover advantage through this approval. Everolimus is an mTOR inhibitor that works by blocking signaling pathways involved in tumor growth and angiogenesis. In clinical trials, everolimus showed promising efficacy in stabilizing tumor burden and reducing growth in patients with VHL-associated RCC. Roche further strengthened its market position through an aggressive marketing campaign highlighting the benefits of a targeted therapy over existing options like surgery and radiation. They also launched patient support programs to improve access and adherence. These efforts helped establish everolimus as the standard of care for VHL-associated RCC within two years of launch. According to sales forecasts by a leading pharma company, everolimus captured over 60% market share among targeted therapies by 2023.
Similarly, Novartis adopted a rapid launch strategy for their drug Afinitor (everolimus) after it gained FDA approval for treatment of pancreatic neuroendocrine tumors associated with VHL in 2014. Much later, in 2021 they launched several direct-to-consumer advertising campaigns emphasizing lifeline benefits. This helped generate early diagnosis and brand awareness. Novartis also reinforced marketing activities through publication of real-world usage data illustrating tumor control and quality of life advantages. This strengthened Afinitor's clinical profile and differentiated it from first-line chemotherapy.
Segmental Analysis of Von Hippel-Lindau Disease Market
Insights, By Clinical Manifestations, Retinal Hemangioblastomas Lead the Market Share and Continues to Dominate Throughout the Forecast Period.
By Clinical Manifestations, retinal hemangioblastomas contributes the highest market share at 38.20% in 2024 owing to several factors. Retinal hemangioblastomas commonly develop at a young age and their symptoms like vision loss are quite noticeable to patients. This leads to early diagnosis and treatment-seeking behavior. Additionally, retinal hemangioblastomas can cause permanent blindness if left untreated, creating a strong sense of urgency to address them. Regular eye checkups help detect small tumors early before they impair vision. Existing treatment options for retinal hemangioblastomas like radiation therapy, laser therapy and cryotherapy are also relatively non-invasive compared to procedures for other manifestations. This makes patients more willing to undergo treatment and continue follow-up care. Moreover, retinal hemangioblastomas often occur bilaterally requiring treatment for both eyes, further augmenting market demand. The high clinical severity and positive outcomes with early intervention drive sustained healthcare utilization for retinal hemangioblastomas as the leading clinical manifestation segment.

Insights, By Diagnosis, MRI Dominates as Demand is Growing for Advanced Diagnostic Capabilities.
By Diagnosis, MRI contributes the highest market share of 70.80% in 2024 due to its superior diagnostic accuracy compared to other modalities. MRI allows high resolution visualization of both soft tissues and blood vessels without ionizing radiation. It is particularly a valuable for evaluating central nervous system hemangioblastomas which represent a major site of VHL disease involvement. MRI aids in determining tumor size and their location. It effectively detects even very small and asymptomatic CNS lesions to guide appropriate therapeutic decisions. MRI's non-invasive nature also encourages compliance with regular long-term monitoring. Additionally, MRI has advanced capabilities like diffusion-weighted imaging and susceptibility-weighted imaging that help differentiate hemangioblastomas from other pathologies. Such advanced diagnostic insights enabled by MRI drives its dominant use over alternatives like CT scans for VHL diagnosis and management.
Insights, By End User, Need for Centralized Specialty Care Encourage Hospitals to Develop Advanced Infrastructure
By End User, hospitals contribute the highest share of the market in 2024 owing to their ability to offer centralized specialty care services required for VHL disease patients. Hospitals have dedicated VHL clinics staffed with multidisciplinary teams of specialists including ophthalmologists, neurosurgeons, radiologists and oncologists experienced in the condition’s complex management. This centralized care approach facilitates streamlined coordination among specialties and accurate disease monitoring over the long-term course. Hospitals also have advanced imaging facilities like high field MRI required for complex diagnostic evaluations. They provide a wide range of treatment options under one roof including surgery, radiation therapy, targeted drug therapies and genetic counseling services. Such centralized specialty care enables improved patient outcomes and convenience which attract higher patient volumes to hospitals compared to other end users.
Additional Insights of Von Hippel-Lindau Disease Market
Merck’s WELIREG remains the only approved drug specifically for VHL-related conditions, including renal cell carcinoma (RCC), and is under further investigation for other VHL-associated conditions. This drug approval has been pivotal in driving market growth.
Competitive overview of Von Hippel-Lindau Disease Market
The major players operating in the Von Hippel-Lindau Disease Market include Merck, Novartis, Roche, Exelixis, Bayer, Bedford Laboratories, Direct Therapeutics, Inc, DNAtrix, Astellas Pharma US Inc, CELLECTAR BIOSCIENCES Inc and Burzynski Research Institute, Betta Pharmaceuticals, Reliance Life Sciences, and Lupin.
Von Hippel-Lindau Disease Market Leaders
- Merck
- Novartis
- Roche
- Exelixis
- Bayer
Von Hippel-Lindau Disease Market - Competitive Rivalry, 2024

Von Hippel-Lindau Disease Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Von Hippel-Lindau Disease Market
- August 2021: Merck’s WELIREG (belzutifan) received FDA approval for treating RCC, CNS hemangioblastomas, and pancreatic neuroendocrine tumors linked with VHL disease.
- Early 2023: Novartis is evaluating DFF332, targeting advanced/relapsed ccRCC and other malignancies, showing potential in early phase trials.
Von Hippel-Lindau Disease Market Segmentation
- By Clinical Manifestations
- Retinal Hemangioblastomas
- CNS Hemangioblastomas
- Renal Cell Carcinoma (RCC)
- Pancreatic Neuroendocrine Tumors
- By Diagnosis
- MRI
- CT-Scan
- Others
- By End User
- Hospitals
- Clinics
- Diagnostic Centers

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
How big is the Von-Hippel Lidau Disease Market?
The Global Von-Hippel Lidau disease market size was USD 301.2 Mn in 2024 and is expected to grow at USD 510.3 Mn by 2031.
What will be the CAGR of the Von Hippel-Lindau Disease Market?
The CAGR of the Von Hippel-Lindau Disease Market is projected to be 7.91% from 2024-2031.
What are the major factors driving the Von Hippel-Lindau Disease Market growth?
The advancements in molecular diagnostics for VHL and increased awareness and research on genetic factors are the major factor driving the Von Hippel-Lindau Disease Market.
What are the key factors hampering the growth of the Von Hippel-Lindau Disease Market?
The limited pipeline activity for new therapies and long duration of clinical trials affecting market growth are the major factor hampering the growth of the Von Hippel-Lindau Disease Market.
What is the leading Clinical Manifestation segment in the Von Hippel-Lindau Disease Market?
Retinal Hemangioblastomas leads the clinical manifestations segment.
Which are the major players operating in the Von Hippel-Lindau Disease Market?
Merck, Novartis, Roche, Exelixis, Bayer, Bedford Laboratories, Direct Therapeutics, Inc, DNAtrix, Astellas Pharma US Inc, CELLECTAR BIOSCIENCES Inc, Burzynski Research Institute, Betta Pharamaceuticals, Reliance Life Sciences, and Bayer are the major players.