

The cardiac ablation devices market is estimated to be valued at USD 2.85 Billion in 2025 and is expected to reach USD 6.54 Billion by 2032, growing at a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032. The increasing prevalence of cardiac arrhythmias and the growing demand for minimally invasive procedures are fueling market growth. Key players are investing in the development of irrigated-tip catheters, cryoablation, laser and ultrasound ablation technologies to improve efficacy and reduce risks associated with procedures.
Market Size in USD Bn
CAGR12.6%
| Study Period | 2025-2032 |
| Base Year of Estimation | 2024 |
| CAGR | 12.6% |
| Market Concentration | High |
| Major Players | Abbott Laboratories, Boston Scientific Corporation, Medtronic plc., Biosense Webster, Inc., AtriCure, Inc. and Among Others |
Market Driver - Rising Cardiovascular Diseases and Atrial Fibrillation
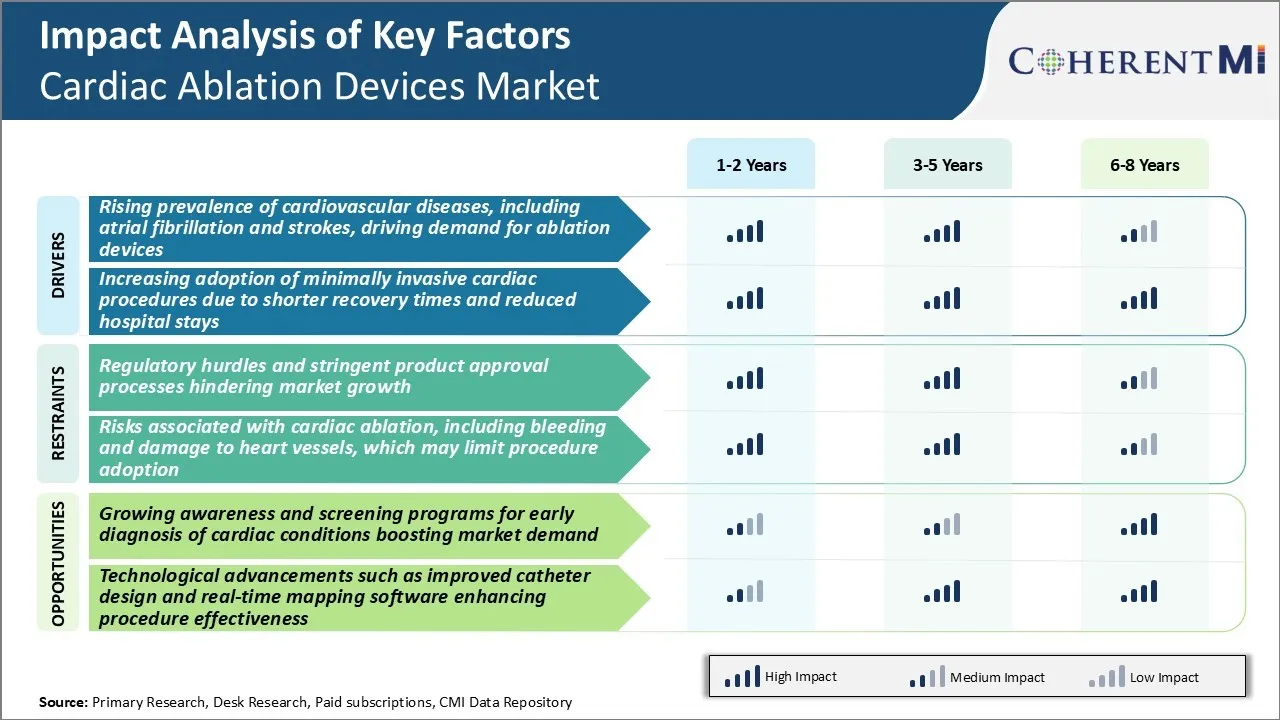
The increasing incidence and prevalence of cardiovascular diseases around the world has become a major public health concern. Cardiovascular diseases are the leading cause of mortality and morbidity globally. According to estimates by the WHO, cardiovascular diseases account for over 17 million deaths each year, which translates to approximately one third of all global deaths. Atrial fibrillation, which is one of the most common types of cardiac arrhythmias, is strongly associated with increased risk of strokes, heart failure and other complications.
Ablation procedures have emerged as an effective treatment option for cardiovascular diseases and atrial fibrillation. Catheter ablation procedure uses targeted delivery of energy to scar or destroy specific areas of heart tissue that are causing abnormal heart rhythms. This helps restore normal heart rhythm and reduces risks of stroke or other complications linked to atrial fibrillation.
As more patients and physicians become aware of the safety and efficacy of ablation procedures compared to prolonged drug therapy or surgical interventions, the adoption of catheter ablation for atrial fibrillation treatment has increased rapidly in recent times. Various new and innovative devices are continuously being developed and marketed by manufacturers to enable improved treatment outcomes for patients through minimally invasive procedures.
Market Driver - Increasing Adoption of Minimally Invasive Cardiac Procedures
While open-heart surgical treatments were conventionally used in the past for treating cardiac arrhythmias and rhythm disorders, minimally invasive endovascular procedures have now become the standard of care. Non-invasive or minimally invasive techniques like catheter ablation offer several advantages over conventional cardiac surgeries such as lesser pain, quicker recovery times, lower risk of surgical complications and scarring.
Patients also experience fewer hospitalization days and can resume normal activities faster after minimally invasive cardiac procedures. With greater awareness about the benefits of reduced invasiveness, more physicians are actively recommending catheter ablation and minimally invasive surgeries to eligible patients who were earlier managed through ongoing drug therapies or open-heart surgeries.
This shift in treatment paradigms globally is a key factor leading to rising demand for advanced cardiac ablation devices used in minimally invasive endovascular procedures. Majority of product innovations in recent times have also been focused on developing compact and user-friendly ablation systems suitable for outpatient or office-based procedures in order to boost patient comfort and compliance to treatment.
As catheter ablation procedures get performed on an increasing number of patients worldwide, the cardiac ablation devices market continues to expand rapidly.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
Market Challenge - Regulatory Hurdles and Stringent Product Approval Processes Hindering Market Growth
The cardiac ablation devices market is facing major challenges due to stringent regulatory policies and long product approval timeframe. The approval process for new cardiac ablation devices involves extensive clinical testing and validation which makes the overall approval process quite lengthy sometimes taking 2-3 years for approval.
Additionally, regulatory policies are frequently updated to ensure patient safety which demands continual product modifications and re-testing. This increases the cost and timeline for manufacturers to comply with the changing regulations.
Moreover, differing regulatory standards across regions like North America, Europe, Asia Pacific etc. means devices need to undergo multiple approval cycles to be marketed worldwide. The lengthy and high cost associated with navigating the complex regulatory ecosystem has made companies skeptical about investing heavily in innovation for new cardiac ablation technologies.
This regulatory bottleneck is hindering the pace of new product introductions and innovations which is critical for market growth. Overall, the strict regulatory compliance requirements and inconsistent standards across regions pose major challenges for the cardiac ablation devices industry.
Market Opportunity - Growing Awareness and Screening Programs for Early Diagnosis of Cardiac Conditions
The market potential for cardiac ablation devices is witness growth due to rising disease awareness and focus on early detection of cardiac arrhythmias. Various initiatives by governments and healthcare organizations aim to promote awareness about signs and risks of atrial fibrillation, atrial flutter and other cardiac arrhythmias.
Large-scale screening of at-risk population like geriatric, diabetic patients is helping in early diagnosis. For example, screening programs in Japan resulted in over 5 times increase in AF diagnosis over last decade. Early detection allows for intervention at mild or asymptomatic stages through minimally invasive ablation procedures. It prevents disease progression to advanced stages necessitating risky surgeries.
The growing acceptance of cardiac ablation as front-line treatment for arrhythmias compared to long-term drug therapy is also fueling demand. Additionally, favorable reimbursement for ablation procedures in developed markets incentivizes adoption.
Thus, increased health awareness, focus on early detection and growing preference for cardiac ablation as first line treatment present significant growth opportunities for market players.
Strategy #1: Product innovation and launches
Key players have consistently focused on developing innovative and advanced cardiac ablation devices to treat AFib and other cardiac arrhythmias. For example, in 2020, Medtronic launched the Cryoballoon Therapy for AFib, an innovative balloon-based catheter ablation technology. This catheter uses argon gas and liquid nitrogen to ablate tissues, enhancing safety and efficacy. Clinical trials showed the therapy provided superior results and ventricular rate control vs drugs alone.
Strategy #2: Acquisitions to enhance product portfolio
Companies have strengthened their position via strategic acquisitions. For example, in 2016, Johnson & Johnson acquired Abbott's heart device business including its cardiac ablation and monitoring products for $25 billion. This expanded J&J's portfolio in the fast-growing cardiac devices market. Similarly, in 2019, Boston Scientific acquired BTG for $4.2 billion to add its vascular intervention and venous insufficiency treatment products.
Strategy #3: Increased R&D investments and collaborations
Players consistently invest higher funds in R&D to develop path-breaking technologies. For example, Abbott invested 15-17% of its net sales in R&D between 2015-2020, helping launch innovative products annually. Abbott also partners with academic institutions and start-ups to co-create new technologies. Such heavy investments and collaborations have boosted players' leadership in the market.
 To learn more about this report, Download Free Sample Copy Insights, By Technology: The Prevalence of Radiofrequency Ablation Technology
To learn more about this report, Download Free Sample Copy Insights, By Technology: The Prevalence of Radiofrequency Ablation Technology
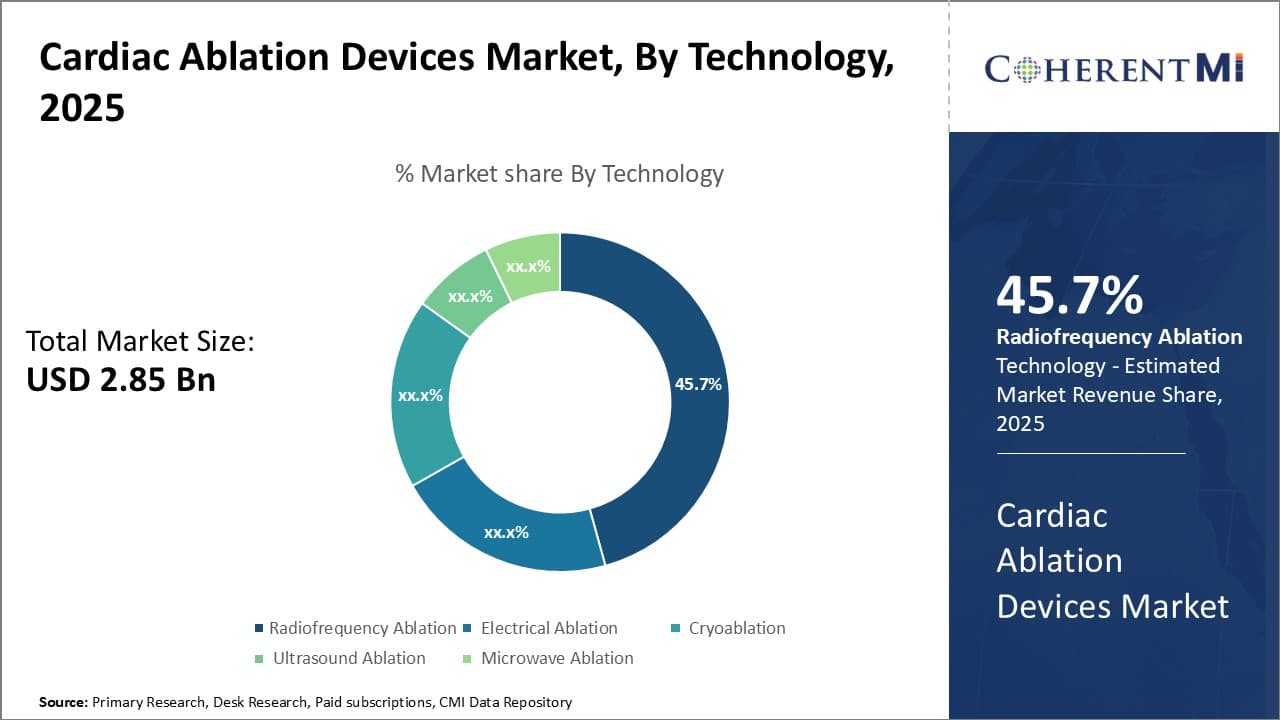
In terms of technology, radiofrequency ablation contributes the highest share of the market owing to its effectiveness and extensive usage in a variety of cardiac procedures. Radiofrequency ablation is a minimally invasive treatment that uses heat generated by radio waves to scar or destroy small areas of heart tissue that cause irregular heartbeats. It has become the mainstay treatment for numerous cardiac arrhythmias given its ability to precisely target aberrant electrical pathways.
Radiofrequency uses low-energy radio waves transmitted through catheter tipped electrodes to heat and kill unwanted tissue. This allows for more controlled tissue damage than other technologies and minimizes risks of complications. The precision and efficacy of radiofrequency make it highly suitable for complex ablation cases and enables treatment of arrhythmias previously considered inoperable.
Its ability to treat hard to reach areas of the heart have driven widespread adoption among electrophysiologists. Moreover, advancements in 3D mapping systems have enhanced the safety and effectiveness of radiofrequency, further cementing its status as the preferred technology.
Insights, By Application: Dominance of Radiofrequency Ablation in Cardiac Rhythm Management
In terms of application, cardiac rhythm management contributes the highest share due to radiofrequency ablation's unparalleled role in treating arrhythmias. Cardiac ablation devices are extensively used in cardiac rhythm management to treat various arrhythmias through elimination or control of aberrant electrical pathways. Within this segment, radiofrequency ablation devices dominate as they are the standard of care treatment for most arrhythmias including atrial fibrillation, atrial flutter, ventricular tachycardia and others. Being minimally invasive, yet highly effective, radiofrequency ablation has revolutionized the management of cardiac rhythm disorders. It provides improved quality of life for patients by restoring normal sinus rhythm and reducing symptoms. The non-invasive nature avoids risks associated with invasive surgeries, making it safer and more desirable than other treatment options. These advantages coupled with the precision it affords electrophysiologists have strengthened radiofrequency ablation's primacy in the cardiac rhythm management segment.
 To learn more about this report, Download Free Sample Copy
To learn more about this report, Download Free Sample Copy
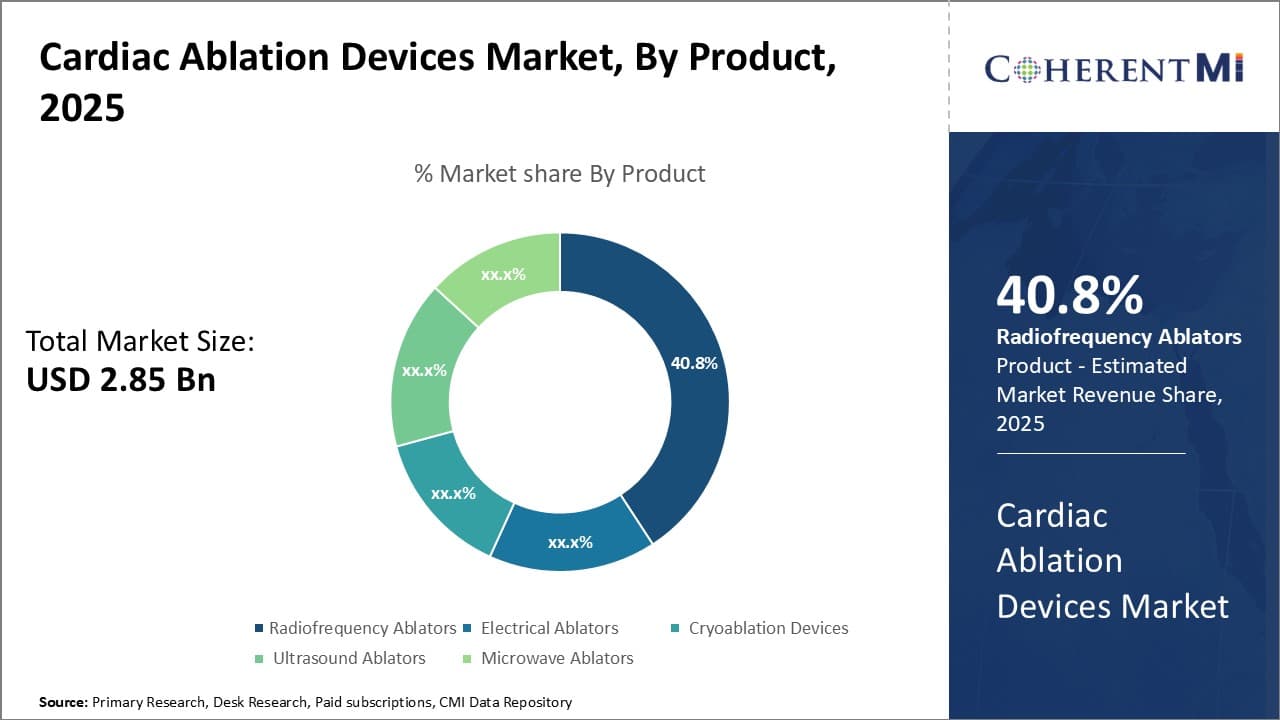
Insights, By Product: Supremacy of Radiofrequency Ablators in the Product Market
In terms of product, radiofrequency ablators contribute the highest share owing to being the technological workhorse of cardiac ablation procedures. Radiofrequency ablation devices form the backbone of cardiac ablation therapies for treating numerous arrhythmias. Radiofrequency ablators are the mainstay tools used by electrophysiologists to administer lesion creation using radiofrequency energy during catheter ablation procedures.
A wide array of radiofrequency ablator products exists with technological advancements focusing on delivering more effective, safer treatments. Irrigated-tip radiofrequency ablators improve lesion quality by preventing coagulum formation. 3D non-contact mapping systems enhance safety and efficacy of radiofrequency ablations.
Robotic systems provide greater stability and control. Such product innovations have strengthened radiofrequency ablators' lock over this segment. Their ability to treat diverse arrhythmogenesis, minimal risk profile and ease-of-use have simplified complex ablation cases, boosting their popularity among healthcare professionals.
As a result, radiofrequency ablators account for the lion's share of the cardiac ablation devices market in the product category.
The major players operating in the Cardiac Ablation Devices Market include Abbott Laboratories, Boston Scientific Corporation, Medtronic plc., Biosense Webster, Inc., AtriCure, Inc., Avanos Medical, Inc., Auris Health, Inc., Olympus, CONMED Corporation, ERBE ELEKTROMEDIZIN GMBH, AngioDynamics, Teleflex Incorporated, St. Jude Medical Inc., Lepu Medical Technology (Beijing) Co., Ltd., and MicroPort Scientific Corporation.
Would you like to explore the option of buying individual sections of this report?
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Cardiac Ablation Devices Market is segmented By Technology (Radiofrequency Ablation, Electrical Abla...
Cardiac Ablation Devices Market
How big is the cardiac ablation devices market?
The cardiac ablation devices market is estimated to be valued at USD 2.85 Billion in 2025 and is expected to reach USD 6.54 Billion by 2032.
What are the key factors hampering the growth of the cardiac ablation devices market?
The regulatory hurdles and stringent product approval processes are primarily hindering market growth. Furthermore, risks associated with cardiac ablation, including bleeding and damage to heart vessels, which may limit procedure adoption are among the major factors hampering the growth of the cardiac ablation devices market.
What are the major factors driving the cardiac ablation devices market growth?
The rising prevalence of cardiovascular diseases, including atrial fibrillation and strokes, are driving demand for ablation devices. In addition, increasing adoption of minimally invasive cardiac procedures due to shorter recovery times and reduced hospital stays are among the major factors driving the cardiac ablation devices market.
Which is the leading technology in the cardiac ablation devices market?
The leading technology segment is radiofrequency ablation.
Which are the major players operating in the cardiac ablation devices market?
Abbott Laboratories, Boston Scientific Corporation, Medtronic plc., Biosense Webster, Inc., AtriCure, Inc., Avanos Medical, Inc., Auris Health, Inc., Olympus, CONMED Corporation, ERBE ELEKTROMEDIZIN GMBH, AngioDynamics, Teleflex Incorporated, St. Jude Medical Inc., Lepu Medical Technology (Beijing) Co., Ltd., and MicroPort Scientific Corporation are the major players.
What will be the CAGR of the cardiac ablation devices market?
The CAGR of the cardiac ablation devices market is projected to be 12.6% from 2025-2032.