Medullary Thyroid 암 약 시장 규모 및 점유율 분석 - 성장 추세 및 예측 (2024 - 2031)
Medullary Thyroid 암 약 시장은 치료 관 (상 I, Phase II, Preclinical), 관리 루트 (Oral, Subcutaneous, Intravenous, Topical), Molecule 유형 (Monoclonal 항체, 작은 분자, 펩티드,....
Medullary Thyroid 암 약 시장 크기
USD 기준 시장 규모 Mn
CAGR10.6%
| 연구 기간 | 2024 - 2031 |
| 추정 기준 연도 | 2023 |
| CAGR | 10.6% |
| 시장 집중도 | Medium |
| 주요 플레이어 | Bayer 건강 제품정보, TYK 의학, Inc, 적용된 약제 연구분야, 회사 소개, 엘리 릴리와 회사 및 기타 |
저희에게 알려주세요!
Medullary Thyroid 암 약 시장 분석
medullary 갑상선 암 약 시장은 추정됩니다 2024년 USD 156 Mn 견적 요청 2031년 USD 315 Mn화합물 연례 성장률에서 성장하는 (CAGR) 2024에서 2031로 10.6%의.
시장은 지난 몇 년 동안 긍정적 인 성장 추세를 목격하고있다. 전 세계적으로 medullary 갑상선암의 증가가 증가하고 표적 치료 약을 위한 일어나는 수요는 시장에 몰리는 중요한 요인의 몇몇입니다.
Medullary Thyroid 암 약 시장 트렌드
시장 드라이버 - 조기 진단에 대한 인식에 Thyroid 암 및 상승의 전임 증가
갑상선 암 약물 시장은 전 세계의 갑상선암의 상승 발생에 대한 증가를 목격하는 성장입니다. 갑상선암의 전분은 지난 수십 년 동안 실질적으로 상승했으며, 주로 비만의 성장 수준으로 인해 생식 패턴의 변화 및 기타 라이프 스타일 요소가 변화합니다.
전문가의 추정에 따라, 갑상선암의 발현률은 1990년부터 미국에서 거의 3배나 갑상선암이 이제 가장 일반적인 마비율입니다. 이 붓기 임신에 대한 정확한 이유가 불분명하고 개선 된 진단 방법 및 고도로 스크리닝은 또한 암을 검출하는 역할을합니다.
더 중요한 것은 사회 의식이 일어나고 암의 조기 탐지에 대한 사람들의 이해가 있습니다. 디지털 미디어를 통해 정보를 강화하고 예방 의료에 중점을 둔 많은 개인은 정기적인 건강 검진을 위해 선택됩니다. 이것은 치료가 훨씬 더 나은 결과를 얻을 때 예비 단계에서 갑상선 종양을 감지하는 의료 실무자를 가능하게합니다.
또한, 자발적인 조직은 갑상선암의 징후와 증상에 대해 더 높은 위험에 대해 사람들에게 알리기 위해 지역 사회의 다양한 인식 드라이브와 모험 프로그램을 수행하고 있습니다.
Market Driver - Targeted Therapies의 발전
갑상선 암 약물 풍경은 지속적인 변화 연구 활동에 주목할만한 변화입니다. Scientists는 더 높은 efficacy 및 개량한 tolerability를 가진 진화 더 새로운 표적 처리 접근법에 타이어가 없게 작동됩니다.
활성 연구의 주요 영역은 특히 RET proto-oncogene과 방해하는 표적 치료 약을 포함합니다, medullary 갑상선암의 많은 경우에 있는 중요한 운전사. 몇몇 RET 억제물은 임상 시험의 진보된 단계에 있고 이 암 이하 유형과 관련된 유일한 열량 mutations를 해결하기 위하여 잠재력을 붙듭니다.
또한, BRAF 억제제, VEGF/VEGFR 억제제, 면역 검수원 억제제 및 epigenetic 변조기와 같은 다른 표적 형태는 조사의 밑에 또한 입니다. 연구 조직은 전 세계적으로 협력적으로 광범위한 바이오 마커 분석 및 번역 연구를 수행하여 심층적 인 통찰력을 medullary 갑상선암의 분자 병리학으로 얻는다. 다음 세대 수질과 같은 정교한 도구를 사용하여 연구자들은 환자의 독특한 종양 프로파일과 임상 페형에 맞춤화된 맞춤형 치료를 개발하는 데 노력하고 있습니다.
제약 회사는 개방 혁신 컨소시엄 및 공공 개인 파트너십과 같은 혁신적인 모델을 통해 이러한 중요한 연구 endeavors를 촉진하기 위해 크기가 큰 투자를하고 있습니다. 이러한 노력은 앞으로 몇 년 동안의 갑상선암 관리를 크게 개선할 수 있는 새로운 치료 패러다임으로 이어질 것으로 예상됩니다.
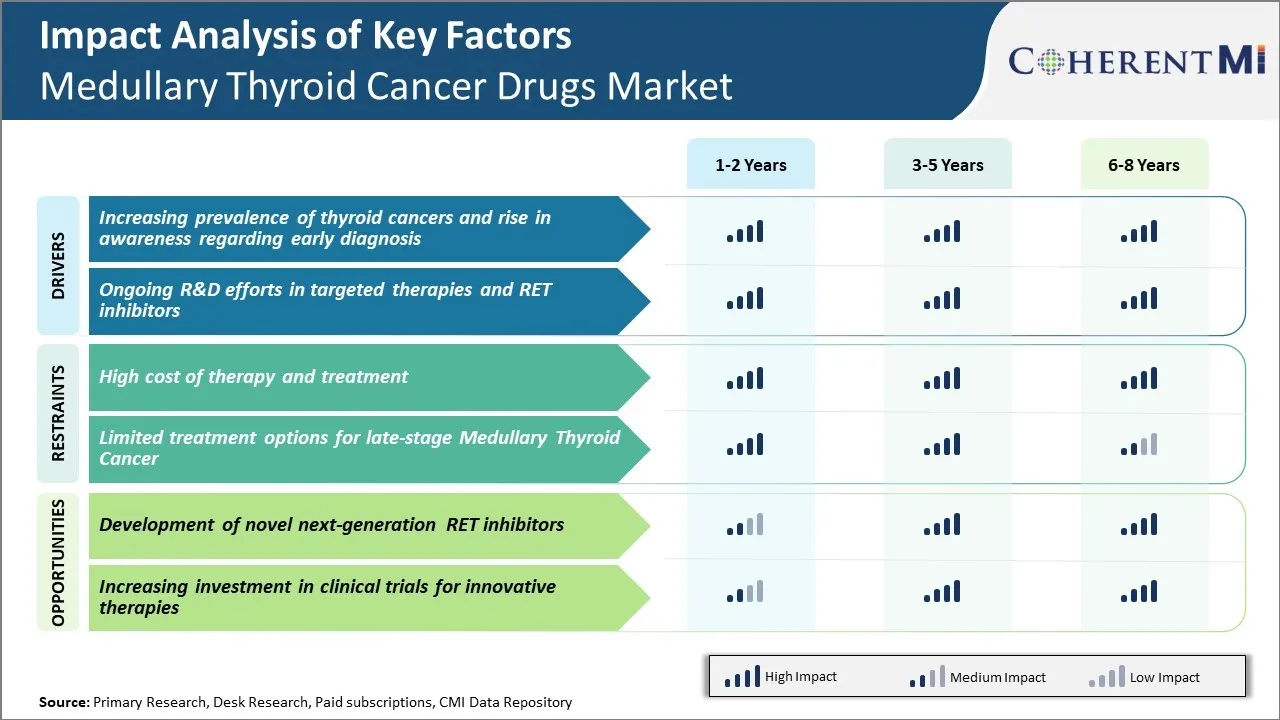
시장 도전 - 치료 및 치료의 높은 비용
치료와 치료의 높은 비용은 medullary 갑상선 암 약 시장의 성장을 위해 중요한 도전을 포즈. 선진 MTC 치료를 위해 사용되는 바닐 디타니, Cabozantinib 및 lenvatinib와 같은 대상 약물 치료는 매우 높은 가격 태그와 함께 제공, 때로는 개인에 대해 6 수치를 초과. 이 뿐만 아니라 환자에 대 한 감당성에 영향을 미칩니다 뿐만 아니라 공공 및 민간 건강 보험과 재투자 협상에 장애물을 입증.
Socio-economic disparities는 다른 지역과 국가 전체에 걸쳐이 비싼 약의 접근 및 섭취에 영향을 미칩니다. 또한 MTC 환자에 필요한 장기 치료는 그들에 더 재정적인 긴장을 뒀습니다. 의약품의 높은 금융 독성에 관한 문제 해결은 제약 회사에서 중요한 것이 더 큰 채택을 촉진하고 향후 몇 년 동안 안정적인 시장 위치를 유지합니다.
시장 기회 - 노벨 차세대 RET Inhibitors 개발
새로운 차세대 RET 억제제의 개발은 medullary 갑상선 암 약 시장에서 플레이어의 주요 성장 기회를 제공합니다. MTC 케이스의 약 60 %는 통제되지 않은 세포 부문에서 결과 RET proto-oncogene의 mutations로 인한 것입니다.
현재 약물은 일반적으로 RET-altered MTC를 치료하는 효과가 있지만 전반적인 응답률은 여전히 약 30-50%에 남아 있습니다. 더 높은 응답률을 달성 할 수있는 더 새로운 RET 억제제를 개발하기위한 범위뿐만 아니라 첫 번째 라인 치료에 대한 저항을 극복 할 수 있습니다.
회사는 선택적인 작은 분자 RET tyrosine kinase 억제물 및 RET 표적 항체 약 conjugate를 탐구하는 연구에서 투자하고 있습니다. 이러한 파이프라인 약물 중 일부는 초기 임상 연구에서 encouraging 결과를 보였습니다. 성공적인 승인 및 상용화는 치료 옵션을 확장하고 시장 참가자에게 상당한 수익을 창출 할 것입니다.
처방자의 선호도 Medullary Thyroid 암 약 시장
Medullary 갑상선 암 (MTC) 치료는 진단에 질병 단계에 근거를 둡니다. 초기 단계 MTC를 위해, 총 갑상선ectomy는 표준 첫번째 선 처리입니다. 암이 로컬 림프 노드 (단 III)로 확산되면, 처방전은 방사성 요오드 ablation 약 I-131 (Azedra, Novartis) 포스트-surgery를 추천합니다.
고급 또는 메타 정적 MTC (단 IV)에 대 한, 첫 번째 선 시스템 치료 일반적으로 대상 키아제 억제제를 포함. Cabozantinib (Cabometyx, Exelixis), RET, VEGFR2 및 MET 수용체를 막는 멀티 키아제 억제제는 피벗 시험에서 보이는 개량한 진행 자유로운 생존에 근거를 둔 선호한 선택권이 되었습니다. 일반적으로 사용되는 다른 RET 억제제는 vandetanib (Caprelsa, AstraZeneca) 및 selpercatinib (Retevmo, Eli Lilly)를 포함합니다.
첫 번째 라인 옵션에서 질병이 진행될 때, 처방전은 pralsetinib (Gavreto, Blueprint Medicine) 또는 화학 요법과 같은 다른 대상 약물을 고려할 수 있습니다. 퇴행성 MTC를 위해, 임상 시험은 LOXO-292 (Loxo Oncology)와 같은 더 새로운 조사 대리인을 증발해서 환자의 분자 단면도 및 prognosis에 근거를 두어 고려될지도 모릅니다.
치료 선택에 대한 주요 영향은 환자의 증상, 종양 마커 레벨, 사전 치료 역사, comorbidities 및 유전적 mutations 검출. 질병 통제를 극화하는 동안 Minimizing 처리 부작용은 높은 prescribing 우선권입니다.
치료 옵션 분석 Medullary Thyroid 암 약 시장
Medullary 갑상선 암은 4 단계로 나눌 수 있습니다 - I, II, III 및 IV - 종양 크기와 확산을 기반으로합니다. 갑상선에 confined 단계 I/II 질병을 위해, 갑상선 (thyroidectomy)를 제거하는 수술은 1 차적인 처리 선택권입니다. 암이 목에 림프 노드로 확산되는 단계 III 질병을 가진 환자를 위해, 갑상선ectomy와 함께 더 광대한 수술 (변경된 목)는 추천됩니다.
symptomatic 또는 진보적인 단계 IV 질병을 위해 암은 몸의 다른 부속에 metastasize가 있는, 약 치료 선호됩니다. 가장 통용되는 약의 2개는 vandetanib와 cabozantinib입니다. Vandetanib (Caprelsa)는 종양 성장과 확산에 관련된 효소의 일부를 차단하여 작동하는 tyrosine kinase 억제물입니다. symptomatic 또는 진보적인 medullary 갑상선 암을 위한 선호됩니다. Cabozantinib (Cometriq/Cabometyx) 다른 tyrosine 키아제 억제제는 medullary 갑상선암을 몰기 위하여 알려지는 RET 단백질을 막습니다.
창의적인 배려를 위해, 화상 진찰 시험과 외과 제거에 의하여 metastatic 병변의 지방화 또는 열 병변 절차는 선택 케이스에 있는 증후를 제공합니다. 외부 광속 방사선 치료는 또한 지방화된 뼈 또는 뇌 대사를 통제하는 것을 도울지도 모릅니다.
주요 플레이어가 채택한 주요 승리 전략 Medullary Thyroid 암 약 시장
표적 약물 개발에 초점: Bristol-Myers Squibb, Exelixis 및 Takeda와 같은 리드 플레이어는 MTC를 치료하기 위해 대상 약물 개발에 대한 R & D 노력을 집중했습니다. Bristol-Myers Squibb는 Cabometyx (cabozantinib), 2016 년에 승인 된 구두 TKI를 개발했습니다. Ret mutation-positive 고급 MTC 환자에서 중요한 항 종양 활동을 전시했습니다. Bristol-Myers Squibb는 상당한 시장 점유율을 얻었다.
In-licensing/partnership 거래: 내부 전문 지식이 부족한 기업은 파트너십과 라이센싱 거래가 있습니다. 예를 들어, 2015 Exelixis에서 특정 territories에 대한 브리스톨 마이어스 Squibb에서 licensed cabozantinib. Exelixis의 개발 비용 및 위험 감소. 그것은 브랜드 이름 Cometriq의 밑에 cabozantinib를 시장에 내놓고 수익을 성장했습니다.
새로운 표시로 확장: 성공적인 약물은 새로운 표시 / 설정에서 사용 확장을 평가하고 있습니다. Cabometyx는 지금 adjuvant와 neoadjuvant MTC 조정에서 공부되고, 성공적인 경우에, 처리에서 더 많은 환자를 붙잡을 수 있었습니다. Exelixis의이 전략은 미국 내의 exclusivity를 잃어버린 후 Cabometyx 판매를 지속했습니다.
목표 약물 개발, 파트너십 및 확장의 이러한 전략은 회사가 규제 승인을 빨리 달성하는 데 도움이, 감소 비용 및 위험, Cabometyx와 같은 약물의 상업적 잠재력을 극대화, 시장 점유율과 수익을 증가하는 선도. 그들은이 공간에서 성공을 위한 표준을 설정했습니다.
세그먼트 분석 Medullary Thyroid 암 약 시장
Insights, Therapeutic Pipeline : 초기 혁신 운전 단계 I 시험
치료 파이프라인의 관점에서, 단계 나는 혁신적인 약물 연구에 높은 투자에 빚지고 시장의 가장 높은 점유율에 기여. 인간 임상 시험에 있는 처음 테스트 단계로, 단계 나는 그들의 안전, tolerability 및 pharmacokinetics를 위한 새로운 분자 entities의 광대한 탐험을 봅니다.
갑상선암의 갑상선암의 갑상선 및 제한된 치료 옵션은 전임상 연구에서 활동의 소설 표적과 메커니즘을 적극적으로 추구하고 있습니다. 약물 입력 단계 나는 일반적으로 기존 승인 치료에 비해 개입을위한 새로운 분자 통로를 제공합니다. 혁신적인 후보자의 초기 평가는 초기 인간 응답을 이해하기 위해 중요한 연구 기금을 유치합니다.
단계 동안 나는 더 높은 투자 비용과 위험이 나중에 단계에 비해, 성공적인 재판은 더 개발을위한 기초를 놓습니다. 연구자들은 또한 미래 예측을 알리기 위해 귀중한 pharmacokinetic 및 pharmacodynamic 통찰력을 얻습니다. 초기 단계의 가능성을 탐구하는 혁신과 의지의 이 문화는 치료 파이프라인 세그먼트 내의 주요 시장 점유율에 가장 기여합니다.
Insights, 관리 루트 : 구강 관리는 환자 수요를 지배
행정의 경로에서, 구두는 편리한 투약 선택권을 위한 강한 환자 수요 때문에 시장의 가장 높은 몫을 공헌합니다. 갑상선 갑상선과 같은 희귀 한 암으로 생활 수많은 의사 방문과 일상적인 일상을 혼란 하는 정맥 주입.
환자는 강하게 의학 감독 없이 가정에 각자 지시될 수 있는 구두 요법을 선호합니다. 이것은 더 큰 융통성을 제공하고, 진료소/병원 여행을 피하고, subcutaneous 주입 또는 정맥 드립과 비교된 생활의 질에 더 적은 가시적 충격이 있습니다. 구두 행정은 또한 환자가 파괴적인 절차 없이 약을 신중하게 가지고 갈 때 더 나은 처리 고착과 관련됩니다.
갑상선암 관리의 만성 성격을 감안하고, 구두 약은 편익과 라이프 스타일에 영향을 미치는 중요한 환자 선호도를 해결함으로써 높은 섭취를 볼 수 있습니다. 이 환자의 요구와 receptiveness oral 옵션은 관리 세그먼트의 경로 내에서 중요한 시장 점유율을 구동한다.

통찰력, Molecule 유형에 의하여: Monoclonal 표적 치료의 앞에 항체
분자 유형의 관점에서, monoclonal 항체는 새로운 표적을 악용하기에 있는 성공 때문에 가장 높은 몫을 공헌합니다. 약제 연구는 medullary 갑상선 carcinogenesis에서 관여된 분자 통로의 매우 강화된 이해가 있습니다.
질병 개발 및 진행과 관련된 여러 드라이버 및 유전자가 식별되었습니다. 이 확장된 분자 지식은 dysregulated 단백질에 대 한 표적된 단일 클론 항체 치료의 개발. 이 종류에 있는 약은 종양에서 교환된 세포 수용체에 있는 호밍에 의해 높은 효험 및 특이성을 보여줍니다.
몇몇 monoclonal 항체는 RET, NTRK 및 VEGF 같이 운전사에 대하여 조기 결과를 달성하는 후에 진보된 임상 발달에서 입니다. 그들의 참신 기계장치에는 찬성된 작은 분자 선택권을 가진 몇몇 overlapping 독성이 있습니다. 기존 치료사가 모노클론 항체 연구와 분자 유형 세그먼트 내의 섭취를 촉진하지 않는 특정 표적을 악용하는 이 진보. 더 많은 성공은 이 세그먼트의 리더십을 강화하고 새로운 대안을 통해 첫 번째로 큰 장점을 제공합니다.
추가 통찰력 Medullary Thyroid 암 약 시장
- Regorafenib와 TY-1091와 같은 각종 약은 임상 발달의 다른 단계에서 입니다. Regorafenib는 특히 Medullary Thyroid 암에 있는 angiogenic와 oncogenic 통로 긴요한 표적, TY-1091는 차세대 RET 억제물 플랫폼의 일부입니다.
- Medullary Thyroid 암은 모든 갑상선 암의 3%-4%에 대해 구성합니다. 약 1,000 개의 새로운 사례는 미국에서 매년 진단됩니다. 이 암은 희귀하지만 종종 다른 갑상선 암 유형보다 공격적입니다.
경쟁 개요 Medullary Thyroid 암 약 시장
Medullary Thyroid Cancer Drugs Market에서 운영하는 주요 선수는 Bayer HealthCare, TYK Medicines, Inc, Applied Pharmaceutical Science, Exelixis, Inc. 및 Eli Lilly 및 Company를 포함합니다.
Medullary Thyroid 암 약 시장 선두
- Bayer 건강 제품정보
- TYK 의학, Inc
- 적용된 약제 연구분야
- 회사 소개
- 엘리 릴리와 회사
Medullary Thyroid 암 약 시장 - 경쟁 경쟁

Medullary Thyroid 암 약 시장
(주요 플레이어가 지배)
(많은 플레이어가 있는 매우 경쟁적)
최근 개발 Medullary Thyroid 암 약 시장
- 7월 2024일, 바이어 건강 Care’s drug Regorafenib는 Medullary Thyroid Cancer (MTC)의 치료를위한 Phase II 임상 시험을 발표했습니다. Regorafenib, 멀티 키아제 억제제, 종양 성장과 관련된 다양한 통로를 대상으로 MTC에 대한 잠재적 치료 옵션을 만들기, 특히 다른 치료에 metastatic 또는 굴절 인 경우. 이 개발은 Bayer의 지속적인 노력으로 표적 암 치료에 초점을 맞추고 있습니다.
- 7월 2024일, TYK 의학, Inc.는 현재 단계 I/II 임상 시험에서 인 그것의 신중한 RET 억제물, TY-1091를 옹호한다는 것을 발표했습니다. 약물은 Preclinical 연구에서 중요한 항 종양 활동을 보여주었으며 특히 RET mutations를 대상으로합니다. 지속적인 시험은 RET-fusion non-small 세포 폐암, RET-mutation medullary 갑상선암 및 다른 RET-altered 종양을 가진 환자에서 TY-1091를 증발하는 것에 집중됩니다.
- 7월 2024일에서, 적용되는 약제 과학은 APS03118, 차세대 선택적 RET 억제제를 개발했다고 발표했다. 그것은 현재 전임상에서, 그리고 그것의 Investigational 새로운 약 (IND) 신청을 위한 FDA 승인을 받았습니다. 또한, APS03118의 임상 응용 프로그램은 National Medical Products Administration (NMPA)의 승인을 받았습니다.
Medullary Thyroid 암 약 시장 세분화
- Therapeutic 파이프 라인
- 단계 I
- 단계 II
- 진료시간
- 행정구역
- 뚱 베어
- 관련 기사
- 한국어
- 제품정보
- Molecule 유형
- Monoclonal 항체
- 작은 분자
- 사이트맵
- 유전자 치료
- 제품 유형
- 한국어
- 주요 특징
- 단청/복합

구매 옵션을 알아보시겠어요?이 보고서의 개별 섹션?
자주 묻는 질문 :
얼마나 큰 메달 갑상선 암 약 시장?
갑상선 암 약 시장은 2024 년 USD 156 Mn에서 평가 될 것으로 예상되며 2031 년 USD 315 Mn에 도달 할 것으로 예상됩니다.
갑상선 암 약 시장의 성장에 대한 주요 요인은 무엇입니까?
치료 및 치료의 높은 비용 늦은 단계의 폐암 치료 옵션은 주요 요인은 폐암 약물 시장의 성장을 엄수하는 주요 요인입니다.
medullary 갑상선 암 약 시장 성장을 운전하는 주요 요인은 무엇입니까?
갑상선암의 증가와 조기 진단 및 진행 R & D 노력에 대한 인식에 상승 표적 치료 및 ret 억제제는 medullary 갑상선암 약 시장을 모는 주요 요인입니다.
갑상선 암 약 시장에서 최고의 치료 파이프라인은 무엇입니까?
주요한 치료 파이프라인 세그먼트는 단계 I.입니다.
medullary 갑상선 암 약 시장에서 작동하는 주요 선수는 무엇입니까?
Bayer 건강 관리, TYK 의학, Inc, Applied Pharmaceutical Science, Exelixis, Inc. 및 Eli Lilly 및 Company는 주요 선수입니다.
갑상선 암 약 시장의 CAGR는 무엇입니까?
갑상선 암 약 시장의 CAGR는 2024-2031에서 10.6%가 될 것으로 예상됩니다.