Triple Negative Breast Cancer (TNBC) Treatment Market SIZE AND SHARE ANALYSIS - GROWTH TRENDS AND FORECASTS (2024 - 2031)
Triple Negative Breast Cancer (TNBC) Treatment Market is segmented By Drug Development Stage (Phase III, Phase II, Phase I, Preclinical/Discovery stag....
Triple Negative Breast Cancer (TNBC) Treatment Market Size
Market Size in USD Mn
CAGR5.3%
| Study Period | 2024 - 2031 |
| Base Year of Estimation | 2023 |
| CAGR | 5.3% |
| Market Concentration | Medium |
| Major Players | Jiangsu HengRui Medicine, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd., Akeso Biopharma, ProLynx, Phoenix Molecular Designs and Among Others. |
please let us know !
Triple Negative Breast Cancer (TNBC) Treatment Market Analysis
The triple negative breast cancer (TNBC) treatment market is estimated to be valued at USD 1000 million in 2024 and is expected to reach USD 1432 million by 2031, growing at a compound annual growth rate (CAGR) of 5.3% from 2024 to 2031. TNBC treatment has gained significant attention in recent years due to the increasing prevalence of breast cancer globally. However, high treatment costs and lack of awareness in low-income countries are some of the factors restraining the market growth.
Triple Negative Breast Cancer (TNBC) Treatment Market Trends
Market Driver - Increasing Incidence of Triple Negative Breast Cancer Globally
The incidence of triple negative breast cancer has been steadily rising across the world in the past few decades. Several epidemiological studies have found higher rates of triple negative breast cancer in younger women less than 50 years of age as compared to other types of breast cancer. Younger women also have poorer survival rates due to more aggressive disease biology and lack of effective targeted therapies.
Furthermore, developing nations are witnessing disproportionately higher incidences particularly among African, Hispanic and Native American women populations who are predisposed to early onset of breast cancer. Experts believe genetic as well as environmental/lifestyle factors like obesity, lack of physical activity, unhealthy diet, alcohol consumption etc. negatively impact the disease risk in these demographics over time.
A recent study estimated over 260,000 new cases and over 75,000 deaths from triple negative breast cancer in 2020 across 184 countries. Global incidences are projected to increase manifold in the coming decades due to above population trends, highlighting the urgent need for development of more effective treatment approaches. This growing patient pool diagnosed with aggressive triple negative breast cancer represents a significant commercial opportunity for novel drugs targeting this high unmet medical need.
Market Driver - Advancements in Immunotherapy and Targeted Drug Delivery Technologies
Scientific advancements in complex fields of cancer immunotherapy and targeted drug delivery have opened promising new avenues for more effective treatment of triple negative breast cancer. Researchers are actively developing diverse modalities to leverage body's natural immune defenses as well as techniques to selectively transport anti-cancer therapies intracellularly.
Cancer immunotherapy relies on enabling the immune system to recognize and destroy cancer cells on its own. Several immunotherapies like checkpoint inhibitors, cancer vaccines, adoptive cell transfer etc. have shown success against other malignancies. Ongoing clinical research is exploring applications of these approaches either as monotherapy or in combination with chemotherapy for TNBC patients. Early results indicate immunotherapy may improve survival when used adjunctively with standard of care regimens. Additionally, factors like high tumor mutational burden in TNBC create an immuno-stimulatory microenvironment more susceptible to immunotherapy interventions.
Research combining antibody-drug conjugates, inorganic nanoparticles, liposomes and other nanocarriers with chemotherapy or targeted agents holds promise. Overall, rapid progress in cancer immunology and nanomedicine is fueling clinical innovation and translational research to design more targeted and well-tolerated TNBC treatment regimens. This is expected to positively impact the triple negative breast cancer (TNBC) treatment market with new therapeutic alternatives in the coming years.
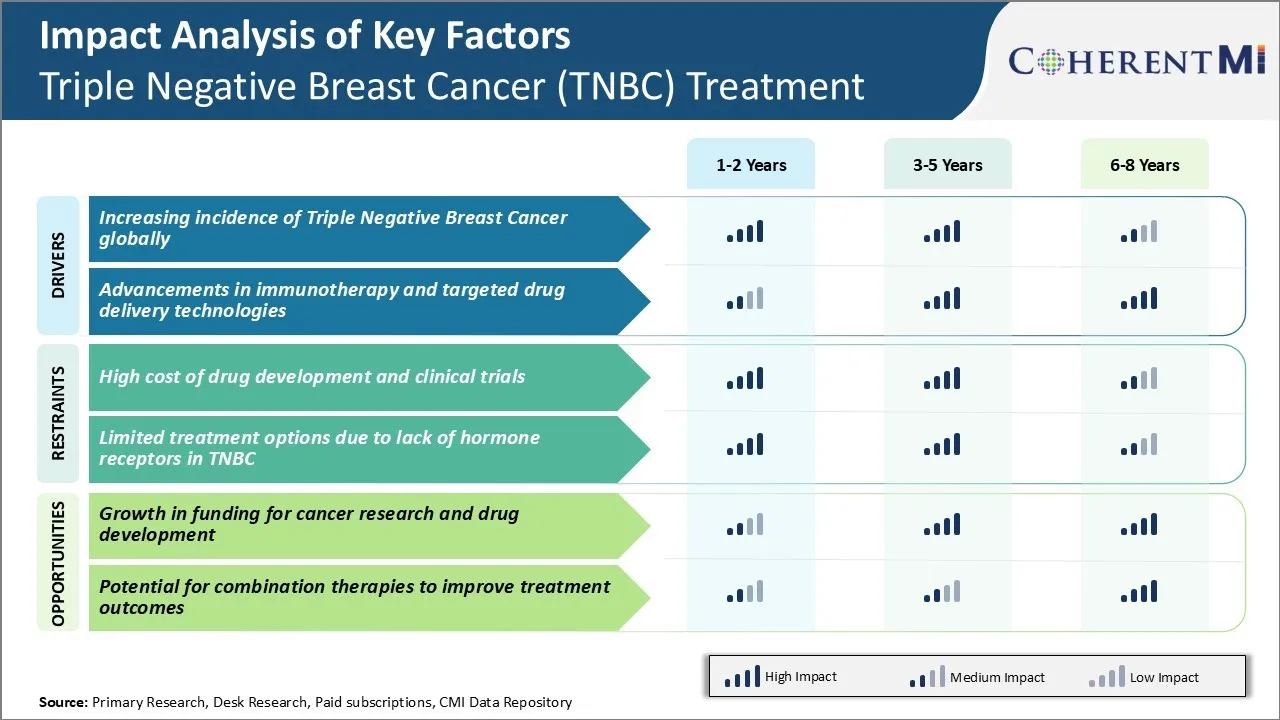
Market Challenge - High Cost of Drug Development and Clinical Trials
One of the major challenges faced by companies operating in the triple negative breast cancer (TNBC) treatment market is the extremely high cost associated with drug development and clinical trials. Developing an entirely new drug from the research stage to final FDA approval typically costs over USD 2 billion.
Specific to TNBC treatments, the development process is further complicated given the heterogeneity and lack of well-defined biomarkers for the disease. This necessitates very large phase 3 clinical trials involving thousands of patients to demonstrate efficacy of an experimental drug. Such late-stage trials alone can cost over $300-500 million per study. Additionally, the failure rate of oncology drugs in phase 3 is as high as 90%, resulting in billions of dollars of losses for companies.
Given the relatively small addressable patient pool for TNBC compared to other breast cancer subtypes, recouping such enormous R&D investments through product sales remains a major challenge. This high-risk, high-cost dynamic has prevented many large pharma players from actively pursuing the triple negative breast cancer (TNBC) treatment market.
Market Opportunity – Growth in Funding for Cancer Research and Drug Development
One key opportunity in the TNBC treatment market is the significant rise in funding availability for cancer research and drug development over the past decade. Both public and private organizations have substantially increased their financial support for advancing scientific understanding of diseases like TNBC and developing novel therapies.
For instance, leading cancer charitable foundations like Breast Cancer Research Foundation and Susan G. Komen have increased their annual research grants to over $100 million collectively. There has also been a parallel surge in venture capital and private equity funding flowing into early-stage oncology biotechs. Such non-dilutive capital has allowed many small companies to advance more TNBC drug candidates into clinical trials.
Additionally, the National Cancer Institute now allocates around $6 billion annually towards cancer research. With ongoing focus on Precision Oncology and targeted therapies, TNBC which lacks well-defined biomarkers is garnering significant funding attention. This availability of capital will help drive further innovation in the triple negative breast cancer (TNBC) treatment market.
Prescribers preferences of Triple Negative Breast Cancer (TNBC) Treatment Market
Triple Negative Breast Cancer (TNBC) is an aggressive form of the disease with limited treatment options. First-line treatment typically involves chemotherapy to shrink tumors prior to surgery. The most commonly prescribed regimens are AC (doxorubicin + cyclophosphamide) and TAC (docetaxel + doxorubicin + cyclophosphamide). After surgery, prescribers may opt for additional chemotherapy such as Taxol (generic name: paclitaxel) to further reduce the risk of recurrence.
For patients with Stage III or IV disease, prescribers consider chemotherapy a primary treatment approach rather than adjuvant therapy. Common drug choices include Taxol, Taxotere (docetaxel), and Abraxane (nab-paclitaxel albumin-bound particles). Response rates to these medications are approximately 30-40%. For advanced or metastatic TNBC with BRCA1/2 mutations, new PARP inhibitors like Lynparza (olaparib) and Zejula (niraparib) have shown encouraging results in clinical trials and are increasingly being prescribed off-label or in expanded access programs.
Other factors known to influence prescribers include patient characteristics like age, menopausal status, tumor biology, and tolerance to side effects. Additionally, availability of clinical trials and access to specialty drugs play a role. While chemo-resistance remains a challenge, recent approvals of immunotherapy drugs like Tecentriq (atezolizumab) provide new hope for treating this aggressive cancer subtype.
Treatment Option Analysis of Triple Negative Breast Cancer (TNBC) Treatment Market
Triple negative breast cancer (TNBC) lacks expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Treatment depends on the stage of cancer:
Stage I-III: The standard treatment is chemotherapy alone or in combination with surgery. Common chemo regimens include AC (doxorubicin, cyclophosphamide) followed by a taxane like docetaxel or paclitaxel. For early stage disease, AC is preferred as it is more effective. AC demonstrates improved survival rates compared to CMF (cyclophosphamide, methotrexate, 5-fluorouracil) or a taxane alone.
Stage III/IV: Combination chemotherapy remains the mainstay for locally advanced or metastatic TNBC. The preferred combination is a platinum salt (carboplatin or cisplatin) with a taxane, especially in the first-line setting. Platinum-based regimens provide higher overall response rates and longer progression-free survival compared to non-platinum regimens. Examples are weekly paclitaxel plus carboplatin or dose-dense AC followed by paclitaxel/carboplatin.
Immunotherapy also plays a key role. Single-agent PD-1/PD-L1 inhibitors like atezolizumab or pembrolizumab are used after failing one or more chemotherapies. TNBC tumors tend to be more immunogenic, allowing them to benefit from checkpoint blockade immunotherapy.
This analytical overview summarized the recommended treatment options for TNBC based on disease stage and line of treatment, including specific chemo regimens and therapies preferred for their improved efficacy outcomes.
Key winning strategies adopted by key players of Triple Negative Breast Cancer (TNBC) Treatment Market
Drug Development Collaboration:
- In 2019, AstraZeneca entered into a clinical collaboration with Daiichi Sankyo to evaluate Enhertu (trastuzumab deruxtecan) in patients with residual TNBC. Enhertu is an antibody drug conjugate (ADC) being investigated for various HER2-expressing cancers. This collaboration allowed both companies to gain clinical evidence for use of Enhertu in treating residual TNBC, a high unmet need area.
- In 2016, Clovis Oncology gained FDA approval for Rubraca (rucaparib) for advanced ovarian cancer. In 2018, based on positive Phase 2 results, it initiated a pivotal registration trial evaluating Rubraca for metastatic TNBC with BRCA mutations.
Targeted Acquisitions:
- In 2018, GlaxoSmithKline acquired Tesaro for $5.1 billion primarily to gain access to Zejula (niraparib), the first and only approved PARP inhibitor for maintenance treatment of ovarian cancer. In 2020, based on positive data GSK initiated a Phase 3 trial testing niraparib in TNBC. This allows GSK to leverage Tesaro's existing clinical evidence and potentially expand Zejula's use to a lucrative new market segment of TNBC.
Strategic Investments:
- In 2020, Daiichi Sankyo invested $250 million in Turning Point Therapeutics focusing on precision oncology medicines. Turning Point was developing repotrectinib for ROS1-altered cancers including TNBC.
Segmental Analysis of Triple Negative Breast Cancer (TNBC) Treatment Market
Insights, By Drug Development Stage: Developing Essential Treatments Ongoing in Phase III
In terms of drug development stage, phase III contributes the highest share of the triple negative breast cancer (TNBC) treatment marketowning to clinical trials demonstrating efficacy. Phase III trials evaluate effectiveness on larger patient populations and provide critical data for regulatory approval. Given the urgent need to advance TNBC treatment options, a large number of therapies are actively progressing through Phase III evaluation.
Several candidate drugs have shown promising response rates and are nearing commercialization, which could transform standards of care. With many Phase III assets addressing high unmet needs, this late-stage pipeline is primarily fueling market growth.
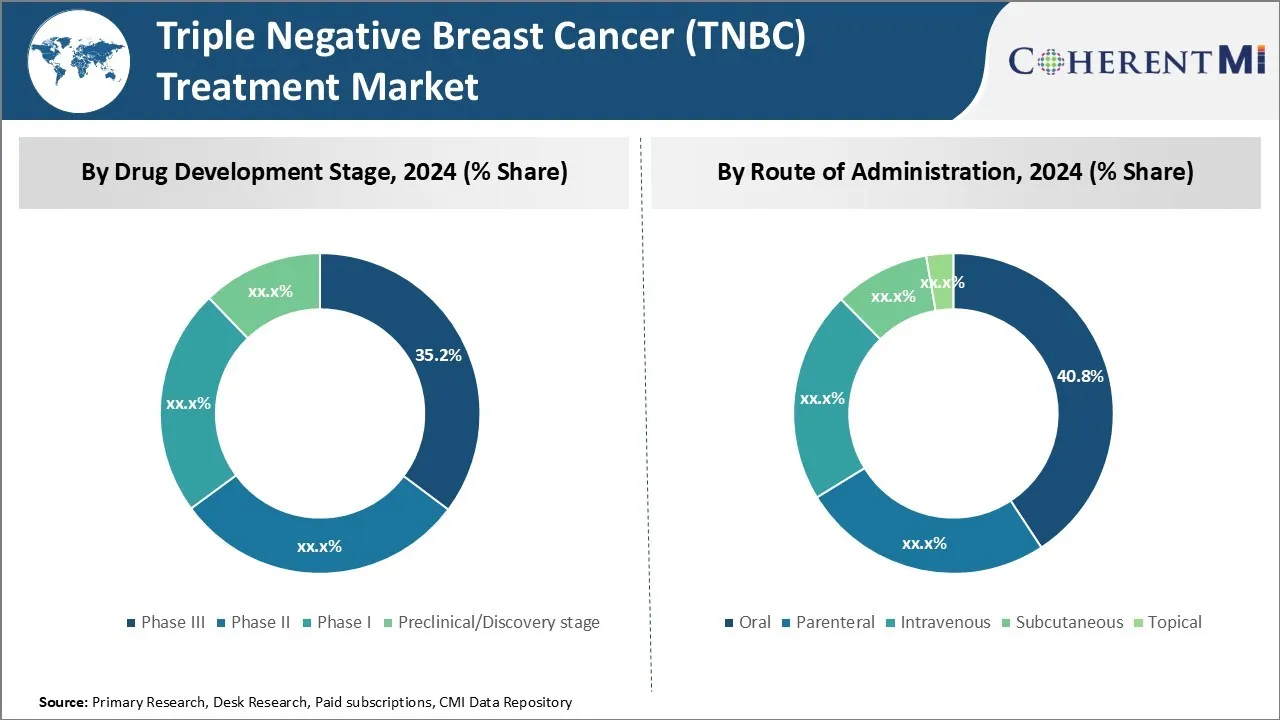
Insights, By Route of Administration: Optimizing Oral Administration Approaches
In terms of route of administration, oral administration contributes the highest share of the triple negative breast cancer (TNBC) treatment market because of preference for convenience. TNBC patients undergo intensive combo treatments which benefit from simplified dosing schedules. Oral administration removes barriers like travel to clinics and time spent receiving infusions.
It also improves adherence and quality of life. Drugmakers are designing new molecular entities with optimized oral profiles to leverage these benefits. The shift toward more user-friendly formulations supports increased patient access and compliance, driving uptake within the oral segment.
Insights, By Molecule Type: Advancing Targeted Mechanisms
In terms of molecule type, monoclonal antibody contributes the highest share of the market due to enhanced selectivity. Monoclonal antibodies allow precision targeting of malignant cells while sparing healthy tissues. They interfere with specific drivers of cancer growth, development and spread.
Continued research expands understanding of immunological pathways in TNBC, pointing to new antibody drug targets. Emerging monoclonal treatments hold promise to transform outcomes by delivering personalized efficacy with improved tolerability. Their mechanistic advantages over traditional small molecules and other modalities create investor interest in developing innovative antibody-based therapies for this disease.
Additional Insights of Triple Negative Breast Cancer (TNBC) Treatment Market
- Triple Negative Breast Cancer (TNBC) affects patients without estrogen, progesterone, or HER2 receptors, limiting treatment options
- The report covers 80+ pipeline drugs under various stages of development, offering hope for expanding future treatment options.
- The majority of TNBC patients are treated using chemotherapy due to the absence of hormone therapy or HER2-targeted drugs.
Competitive overview of Triple Negative Breast Cancer (TNBC) Treatment Market
The major players operating in the Triple Negative Breast Cancer (TNBC) Treatment Market include Jiangsu HengRui Medicine, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd., Akeso Biopharma, ProLynx, and Phoenix Molecular Designs.
Triple Negative Breast Cancer (TNBC) Treatment Market Leaders
- Jiangsu HengRui Medicine
- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd.
- Akeso Biopharma
- ProLynx
- Phoenix Molecular Designs
Triple Negative Breast Cancer (TNBC) Treatment Market - Competitive Rivalry, 2024

Triple Negative Breast Cancer (TNBC) Treatment Market
(Dominated by major players)
(Highly competitive with lots of players.)
Recent Developments in Triple Negative Breast Cancer (TNBC) Treatment Market
- In June 2024, Trilaciclib functions as an immunomodulatory agent and is used alongside chemotherapy. Its inclusion in TNBC trials is significant because it aims to improve the efficacy of chemotherapy by protecting the bone marrow from toxic effects while also enhancing the patient's immune response. This is crucial for expanding treatment options for TNBC, a particularly aggressive form of breast cancer.
- The drug sacituzumab govitecan (Trodelvy), which uses Antibody-Drug Conjugate (ADC) technology, has indeed received Breakthrough Therapy Designation from the FDA for the treatment of triple-negative breast cancer (TNBC). This designation was granted based on its efficacy in treating patients with metastatic TNBC who have undergone at least two prior treatments. Trodelvy demonstrated a response rate of 31% in a clinical trial for heavily pretreated patients with TNBC, highlighting its potential for patients with limited therapeutic options.
- AK117, a CD47-blocking antibody, has been shown to enhance the phagocytic activity of immune cells, specifically macrophages, against tumor cells. This is achieved by blocking the interaction between CD47 (a "don't eat me" signal) and the SIRPα receptor on phagocytes, which normally inhibits phagocytosis. By inhibiting CD47, AK117 enables macrophages to recognize and engulf tumor cells, which offers significant potential in the treatment of cancers such as Triple-Negative Breast Cancer (TNBC).
Triple Negative Breast Cancer (TNBC) Treatment Market Segmentation
- By Drug Development Stage
- Phase III
- Phase II
- Phase I
- Preclinical/Discovery stage
- By Route of Administration
- Oral
- Parenteral
- Intravenous
- Subcutaneous
- Topical
- By Molecule Type
- Monoclonal Antibody
- Peptides
- Polymer
- Small molecule
- Gene therapy
- By Product
- Mono
- Combination
- Mono/Combination

Would you like to explore the option of buying individual sections of this report?
Frequently Asked Questions :
How big is the triple negative breast cancer (TNBC) treatment market?
The triple negative breast cancer (TNBC) treatment market is estimated to be valued at USD 1000 million in 2024 and is expected to reach USD 1432 million by 2031.
What are the key factors hampering the growth of the triple negative breast cancer (TNBC) treatment market?
The high cost of drug development and clinical trials and limited treatment options due to lack of hormone receptors in TNBC are the major factor hampering the growth of the triple negative breast cancer (TNBC) treatment market.
What are the major factors driving the triple negative breast cancer (TNBC) treatment market growth?
The increasing incidence of triple negative breast cancer globally and advancements in immunotherapy and targeted drug delivery technologies are the major factor driving the triple negative breast cancer (TNBC) treatment market.
Which is the leading drug development stage in the triple negative breast cancer (TNBC) treatment market?
The leading drug development stage segment is phase III.
Which are the major players operating in the triple negative breast cancer (TNBC) treatment market?
Jiangsu HengRui Medicine, Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd., Akeso Biopharma, ProLynx, Phoenix Molecular Designs are the major players.
What will be the CAGR of the triple negative breast cancer (TNBC) treatment market?
The CAGR of the triple negative breast cancer (TNBC) treatment market is projected to be 5.3% from 2024-2031.